Colloids 2.0
Colloid 2.0
We investigate the principles of self-assembled materials by making particles that mimic interactions of atoms and molecules
Kontaktperson: Dr. Peter van Oostrum
In our laboratory we work with Patchy colloids in External fields for Bio-inspired Self organizing materials. Let us shortly explain what we mean by this:
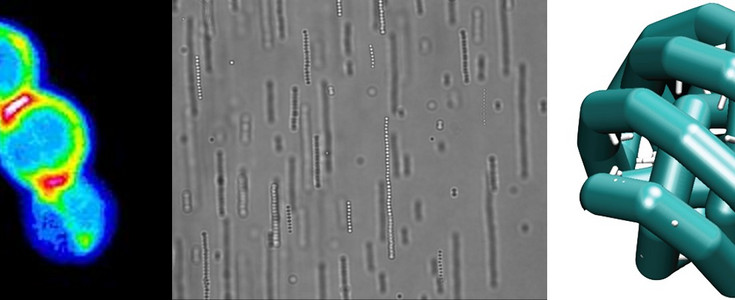
Colloids are chunks of several nm’s to several µm in size of some material dispersed in a fluid. Daily life examples include paints, milk, whipped cream and smoke. Colloids make Brownian (thermal) motion and a colloidal system thus explore the available phase space accessible with an energy budget of about one Boltzmann constant. Ultimately this might allow colloidal systems to reach thermal equilibrium. Often, these thermal equilibriums are ordered, perhaps even crystalline, and therefor this phenomenon is called Self organization. Light microscopy techniques such as video holographic microscopy and confocal microscopy can visualize colloidal phase behaviour in real-time and real-space. This makes colloidal particles ideal building blocks for self-organizing systems that automatically reach certain phases, such as different crystals that may have interesting optical, electrical or mechanical properties. The art of self-organization to produce complex new materials lies in manipulating the interactions between the colloids. When making cheese for instance, one can add enzymes to change the surface chemistry of the protein blobs in milk, change the pH, or add alcohol, to make everything clog together and let the cheese precipitate. One interesting feature of colloids is that their interactions are often dominated by the surface chemistry while the interior of the particles makes them exceptionally susceptible to external fields.
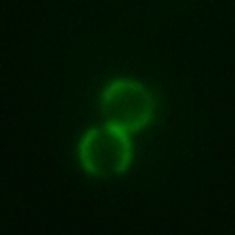
Patchy colloids have different zones, patches, on their surface such that they interact differently in specified directions. This gives rise to interactions analogous to those between atoms and certain molecules such as peptides. Recently these novel interactions attracted significant interest from the scientific community. The simplest example of patchy colloids is that if so-called Janus particles that feature two different faces, like the Roman god after which they are named.
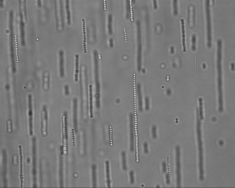
External fields present another way to manipulate the interaction between colloids. In this case forces between the colloids are induced by external fields such as gravity, radiation pressure from bright light, Coulomb forces in electric fields, dielectric forces in electric fields and magnetic forces. In our laboratory we mainly work with AC electric fields and magnetic fields. These fields induce directional interactions between the colloids through induced electric dipoles that tend to promote the formation of linear strings. Colloids can also be made magnetic by incorporation of (superpara)magnetic (iron oxide) nanoparticles. Such particles will string up when you hold a simple permanent magnet close to the sample.