Nanoparticles and membranes
Nanopartikel und Membrane
Interaction of core-shell nanoparticles with membranes. Assembly of core-shell nanoparticles into membranes.
Working on this: Univ. Prof. Dr. Erik Reimhult, Ronald Zirbs and Peter van Oostrum
Cell membranes contain a large part of the delicate machinery of life and comprise the barriers controlling access to and from the interior of the cell. With the increasing use of nanoparticles (NPs) in medical imaging, drug delivery, cosmetics and materials the need is great and increasing to understand how NPs physically interact with cell membranes. On the one hand it is important to understand mechanisms to control risks of novel nanomaterials and to design therapeutic agents which can enter cells specifically and non-destructively. On the other hand, the structure and function of biological membranes inspire development of biomimetic smart materials for biotechnological applications which exploit or are modeled on biological membranes, but given enhanced functionality and external control of properties through incorporation of functional NPs.
One of the primary functions of biological membranes is to provide a two-dimensional barrier which can regulate transport of material from one side to the other. While a barrier is easily created, adding functionality that can selectively in time, space and species move material from one side to the other requires complex machinery in nature. It is a tremendous challenge for human engineering to accomplish the same, but the variety of solutions in biological systems provide inspiration for solutions.
Cell membranes contain a large part of the delicate machinery of life and comprise the barriers controlling access to and from the interior of the cell. With the increasing use of nanoparticles (NPs) in medical imaging, drug delivery, cosmetics and materials the need is great and increasing to understand how NPs physically interact with cell membranes. On the one hand it is important to understand mechanisms to control risks of novel nanomaterials and to design therapeutic agents which can enter cells specifically and non-destructively. On the other hand, the structure and function of biological membranes inspire development of biomimetic smart materials for biotechnological applications which exploit or are modeled on biological membranes, but given enhanced functionality and external control of properties through incorporation of functional NPs.
One of the primary functions of biological membranes is to provide a two-dimensional barrier which can regulate transport of material from one side to the other. While a barrier is easily created, adding functionality that can selectively in time, space and species move material from one side to the other requires complex machinery in nature. It is a tremendous challenge for human engineering to accomplish the same, but the variety of solutions in biological systems provide inspiration for solutions.
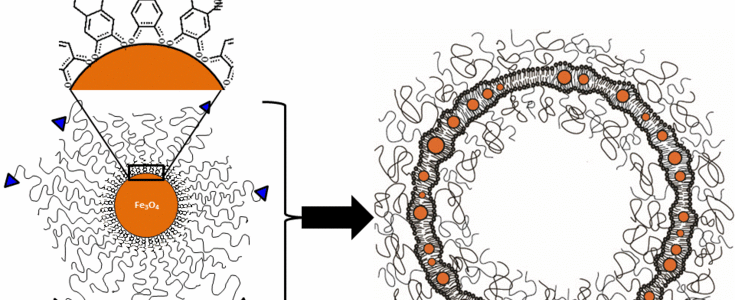
By developing tailor-made magnetic nanoparticles with controlled physico-chemical properties we have building blocks suitable for both probing biological membrane systems and to build our own membrane materials with nanoparticles as our functional components. Using magnetic nanoparticles for this task has the advantage of allowing us to measure their presence and to apply forces to them over large distances also in biological environments like tissues and high ionic strength solutions, where other interaction fields such as light or electric fields are not applicable.
Our work is currently focused in three areas:
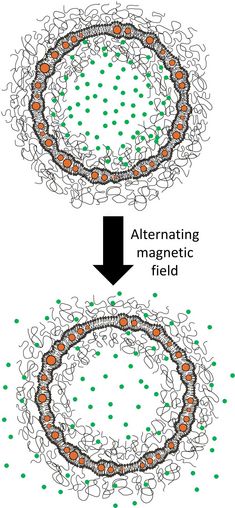
1. Interaction of core-shell nanoparticles with amphiphilic (lipid and polymer) membranes and actuation of biomimetic membranes through magnetic nanoparticles.
2. Developing characterization methods and membrane model systems with which nanoparticle interactions with membranes can be investigated.
3. Self-assembly of core-shell nanoparticles in emulsion systems (oil-water interfaces).
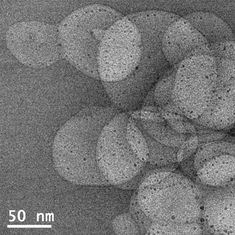
To characterize these systems we employ a range of techniques from high-resolution transmission electron microscopy, chromatography, thermogravimetry analysis, mass spectrometry, interfacial tension measurements as well as optical microscopy, neutron and X-ray scattering.
Selected references:
1. Phospholipase A2-induced degradation and release from lipid-containing polymersomes. M. Mumtaz Virk and E. Reimhult, Langmuir, 34, 395-405 (2018). DOI: 10.1021/acs.langmuir.7b03893
2. Fluorescent Magnetopolymersomes: A Theranostic Platform to Track Intracellular Delivery. O. Bixner, N. Gal, C. Zaba, A. Scheberl and E. Reimhult, Materials, 10, 1303 (2017). DOI: 10.3390/ma10111303
3. Optimization of magneto-thermally controlled release kinetics by tuning of magnetoliposome composition and structure. B. Shirmardi Shaghasemi, M. Mumtaz Virk and E. Reimhult, Scientific Reports, 7, 7474 (2017). DOI: 10.1038/s41598-017-06980-9
4. Magneto-thermal release from nanoscale unilamellar hybrid vesicles. O. Bixner, G. Bello, M. Mumtaz Virk, S. Kurzhals, A. Scheberl, N. Gal, A. Matysik, R. Kraut and E. Reimhult, ChemNanoMat, 2, 1111-1120 (2016). DOI: 10.1002/cnma.201600278
5. Triggered Release from Thermoresponsive Polymersomes with Superparamagnetic Membranes. O. Bixner, S. Kurzhals, M. Virk and E. Reimhult, Materials, 9, 29 (2016). DOI: 10.3390/ma9010029
6. Controlled magnetosomes: Embedding of magnetic nanoparticles into membranes of monodisperse lipid vesicles. O. Bixner and E. Reimhult, Journal of Colloid and Interface Science, 466, 62-71 (2016). DOI: 10.1016/j.jcis.2015.11.071
7. Core-shell nanoparticle monolayers at planar liquid-liquid interfaces: effects of polymer architecture on the interface microstructure. L. Isa, D.C.E. Calzolari, D. Pontoni, T. Gillich, A. Nelson, R. Zirbs, A. Sanchez-Ferrer, R. Mezzenga and E. Reimhult, Soft Matter, 9, 3789-3797 (2013). DOI: 10.1039/C3SM27367A
8. Adsorption of Core-Shell Nanoparticles at Liquid-Liquid Interfaces. L. Isa, E. Amstad, K. Schwenke, E. Del Gado, P. Ilg, M. Kröger and E. Reimhult, Soft Matter, 7, 7663-7675 (2011). DOI: 10.1039/c1sm05407d
9. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. E. Amstad, J. Kohlbrecher, E. Müller, T. Schweizer, M. Textor and E. Reimhult, Nano Letters, 11, 1664-1670 (2011). DOI: 10.1021/nl2001499
10. Electrochemically stimulated release from liposomes embedded in a polyelectrolyte multilayer. N. Graf, F. Albertini, T. Petit, E. Reimhult, J. Vörös and T. Zambelli, Advanced Functional Materials, 21, 1666-1672 (2011). DOI: 10.1002/adfm.201002283