Characterisation of in vitro Membrane-Assisted Protein Synthesis.
Cell-free protein synthesis has hitherto been very successful in the production of soluble proteins. Less successful is the production of viable, correctly-folded membrane proteins. A major obstacle is the tendency for the hydrophobic transmembrane domains to interact and precipitate the products.
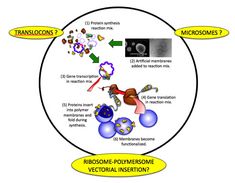
In order to provide them with a hydrophobic shield, artificial lipid and polymer membranes have been used as scaffolds during their production. A powerful method describes the inclusion of artificial membranes during the process of protein translation in the absence of detergents. This in vitro membrane-assisted protein synthesis relies on the insertion of nascent proteins into the artificial membrane while still attached to ribosomes.
Protease digestion assays suggest that the proteins are not only integrated, but are in the correct orientation. In view of the growing interest in this method, it is necessary to understand the mechanism of protein insertion, integration and orientation.
Of interest would be to determine the possible roles of translocons, microsomes and ribosomes in the reaction mix. Ultimately, this would show how an artificial system is able to interact with cell components in order to mimic a highly regulated, complex molecular process.
Contact person: Dr. Darren Tan