Generation and characterization of artificial archaeal cell envelope structures and their relevance as model membrane platforms
[Translate to English:] Generation and characterization of artificial archaeal cell envelope structures and their relevance as model membrane platforms - FWF P29399-B22
[Translate to English:]
Institute for Synthetic Bioarchitectures
Department of NanoBiotechnology, Universität für Bodenkultur Wien
Archaea biology and Ecogenomics Division
Department of Ecogenomics and Systems Biology, Universität Wien
Objective:
The aim of the present project is to gain crucial information on the:
1) optimal procedure to obtain archaeal building blocks, i.e., reproducibly isolated or genetically engineered archaeal S‑layer proteins and archaeal etherlipids,
2) re-construction of archaeal cell envelope structures based on their fundamental building principles and intrinsic self-assembly capability, and
3) biophysical features of these artificial cell envelope models (e.g., permeability, stability, fluidity, etc.) in particular compared to bacterial ones.
The gained knowledge will lead to a straightforward approach for designing artificial model membrane platforms that recapitulate fundamental aspects of membrane biology, in particular the lipid environment, which provides a dedicated reconstitution matrix for membrane-active peptides and transmembrane proteins. Moreover, our worldwide novel work constitutes a highly exciting challenge with impact on basic science such as microbiology, synthetic biology and nanobiotechnology, but also in applied science such as bio-inspired materials for the generation of artificial membrane platforms and the functional coating of medicinal or technically relevant surfaces.
Background:
Many studies on archaea are driven by the intrigue of novel biochemical pathways and the ability of cellular components to function effectively under extreme conditions (e.g., hyper-thermophiles growing at temperatures even higher than 100 °C, acidophiles growing at pH lower than zero and halophilic archaea growing at a salinity of up to 5 M sodium chloride). Particularly halophilic archaea are of special interest for microbiology and nanobiotechnology because of their molecular building principles (Fig. 1). The organization and structure of archaeal cell envelopes may provide the problem solving for issues concerning the mechanical robustness, storage stability or longevity of biomimetic synthetic bioarchitectures based on S‑layer proteins (SLPs). However, archaeal SLPs are in most cases tightly anchored in the cytoplasmic membrane by a hydrophobic domain whereas their bacterial counterparts do not develop a membrane anchor. Therefore, the isolation of archaeal SLPs is more laborious and their reassembly more difficult. Up to now only few studies on the disintegration, isolation and purification of archaeal SLP have been performed so far and a mere of very preliminary data are available. Nevertheless, one of the advantages of archaeal SLPs over bacterial ones is their much larger center-to-center spacing of the morphological units. Recently, archaeal SLPs have also be produced recombinantly. Hence, the controlled self-assembly or recrystallization of isolated archaeal SLPs and, least of all, of recombinantly generated ones is a very challenging task.
In contrast to bacterial membranes where the hydrophilic head group of the lipid is connected by an ester linkage to the hydrophobic tail, archaeal membrane lipids are composed of ether-bonded membrane lipids comprising of isoprenoid chains and a glycerol backbone (Fig. 2). Common structures include the monomeric diphytanyl diglycerol diether, termed archaeol, the dimeric macrocyclic caldarcheol and glycerol dialkyl glycerol tetraetherlipids. The major types of polar head groups found in archaeal intact polar lipids are phosphatidyl and sugar moieties. The higher chemical resistance against hydrolysis, the higher mobility of the lipid molecules (fluidity) over a broad temperature range, and the fact that frequently one monolayer is sufficient to build up a membrane mimics make these archaeal etherlipids to very interesting building blocks. Moreover, the above mentioned features and the generally reduced permeability to ions such as protons and sodium ions predestine reassembled archaeal cytoplasmic membranes as exciting model lipid membrane platforms.
In the present project bacterial building blocks, which have so far utilized for the generation of supramolecular assemblies will be supplemented by isolated and genetically engineered archaeal SLPs and purified archaeal lipid compounds to deliberately modulate the intrinsic biophysical properties of these created novel architectures (Fig. 3). Moreover, it can be anticipated that archaeal SLPs and lipids will show an enhanced structural stability against high temperature, pressure and salt concentration. This achievement would not only tremendously expedite the research on archaeal cell envelope structures but also the biomimetic application of these unique building principles in basic science and in further consequence also in applied science.
Involved people:
Core team: Bernhard Schuster (project leader), Christa Schleper and Simon Rittman (senior scientists), Kevin Pfeifer (PhD student)
Extended scientific stuff: Daniel Birgel, Eva-Kathrin Ehmoser, Uwe B. Sleytr
Related publications:
- Damiati, S., Küpcü, S., Peacock, M., Eilenberger, C., Zamzami, M., Qadri, I., Choudhry, H., Sleytr, U.B., Schuster, B. 2017. Acoustic and Hybrid 3D-Printed Electrochemical Biosensors for the Real-Time Immunodetection of Liver Cancer Cells (HepG2). Biosens. Bioelectron. 94: 500–506. DOI: 10.1016/j.bios.2017.03.045.
- Rodrigues-Oliveira, T., Belmok, A., Vasconcellos, D., Schuster, B., Kyaw, C.M. 2017. Archaeal S-Layers: Overview and Current State of the Art. Front. Microbiol. 8: 2597. DOI: 10.3389/fmicb.2017.02597.
- Damiati, S., Peacock, M., Leonhardt, S., Damiati, L., Baghdadi, M.A., Becker, H., Kodzius, R., Schuster, B. 2018. Embedded Disposable Functionalized Electrochemical Biosensor with a 3D-Printed Flow Cell for Detection of Hepatic Oval Cells (HOCs). Genes 9: 89. DOI: 10.3390/genes9020089.
- Schuster, B. 2018. S-layer protein-based biosensors. Biosensors 8: 40. DOI:10.3390/bios8020040
- Damiatia, S., Peacock, M., Mhannad, R., Søpstad, S., Sleytr, U.B., Schuster, B. 2018. Bioinspired detection sensor based on functional nanostructures of S-proteins to target the folate receptors in breast cancer cells. Sensor Actuator B Chem. 267: 224–230. DOI: 10.1016/j.snb.2018.04.037.
- Mauerhofer, L.M., Reischl, B., Schmider, T., Schupp, B., Nagy, K., Pappenreiter, P., Zwirtmayr, S., Schuster, B., Bernacchi, S., Seifert, A.H., Paulik, C., Rittmann, S.K.M.R. 2018. Physiology and methane productivity of Methanobacterium thermaggregans. Appl. Microbiol. Biot. 102(17): 7643-7656. DOI: 10.1007/s00253-018-9183-2.
- Schuster, B. 2018. S-layer protein-based biosensors. New Biotechnol. 44: S21-S21. DOI: 10.1016/j.nbt.2018.05.143.
- Rodrigues-Oliveira, T., Souza, A.A., Kruger, R., Schuster, B., Maria de Freitas. S., Kyaw, C.M. 2019. Environmental factors influence the Haloferax volcanii S-layer protein structure. PLoS ONE 14(5): e0216863. DOI: 10.1371/journal.pone.0216863.
- Damiati, S., Scheberl, A., Zayni, S., Damiati, S.A., Schuster, B., Kompella, U.B. 2019. Albumin-bound nanodiscs as delivery vehicle candidates: Development and characterization. Biophys. Chem. 251: 106178. DOI: 10.1016/j.bpc.2019.106178.
- Zink, I.A., Pfeifer, K., Wimmer, E., Sleytr, U.B., Schuster, B., Schleper, C. 2019. CRISPR-mediated gene silencing reveals involvement of the archaeal S-layer in cell division and virus infection. Nat. Commun. 10: 4797. DOI: 10.1038/s41467-019-12745-x.
- Czernohlavek, C., Schuster, B. 2020. Formation of planar hybrid lipid/polymer membranes anchored to an S-layer protein lattice by vesicle binding and rupture. Soft Mater. DOI: 10.1080/1539445X.2019.1708753.
- Czernohlavek, C., Schuster, B. 2020. Formation and characteristics of mixed lipid/polymer membranes on a crystalline surface-layer protein lattice. Biointerphases 15: 011002. DOI: 10.1116/1.5132390.
Funding:
Austrian Science Fond (FWF), Project P 29399-B22. Start of project: 1st October 2016. Background information can be retrieved from the FWF pf.fwf.ac.at/de/wissenschaft-konkret/project-finder/= and BOKU: https://www.boku.ac.at/?id=23856
Contact:
Assoc. Prof. Bernhard Schuster, PhD
Institute for Synthetic Bioarchitectures
Department of NanoBiotechnology
Universität für Bodenkultur Wien
Muthgasse 11, 1190 Wien, Austria
e-mail: bernhard.schuster@boku.ac.at
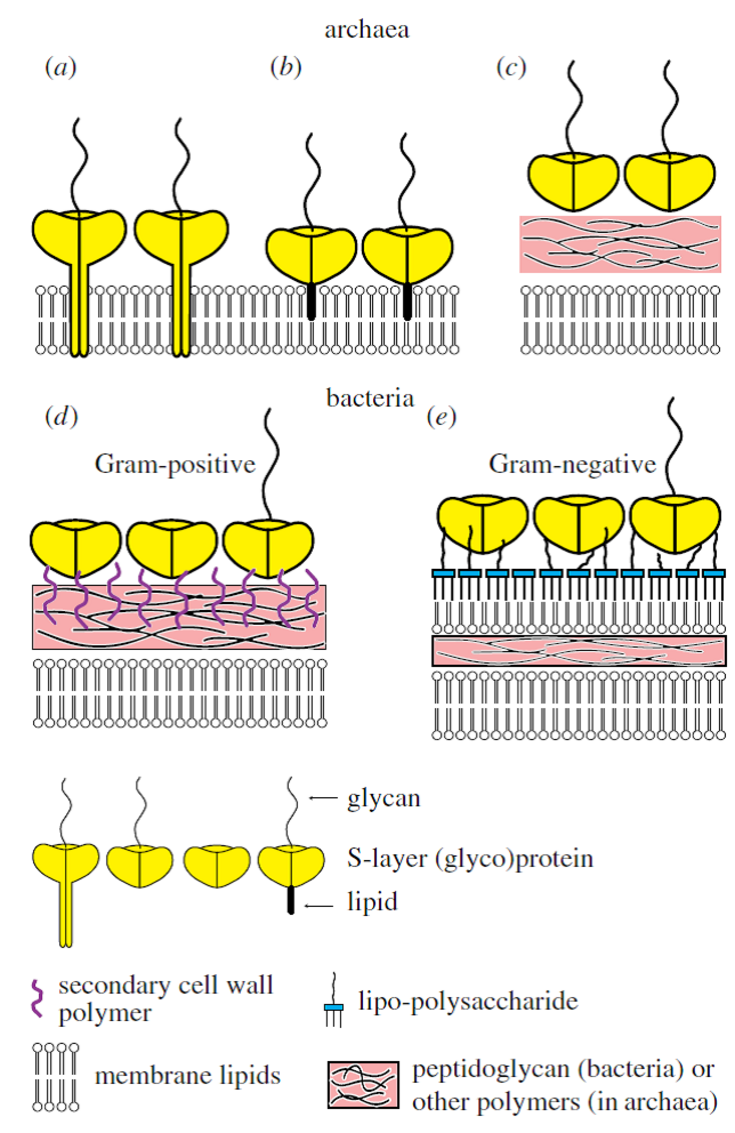
[Translate to English:] Fig. 1. Schematic illustration of the supramolecular architecture of the major classes of prokaryotic cell envelopes containing surface (S)-layers. S-layers in archaea with glycoprotein lattices as exclusive wall component are com-posed either of mushroom-like subunits with pillar-like, hydrophobic transmembrane domains (a), or lipid-modified glycoprotein subunits (b). Individual S-layers can be composed of glyco-proteins possessing both types of membrane anchoring mechanisms. Few archaea possess a rigid wall layer as intermediate layer between the plasma membrane and the S-layer (c). In Gram-positive bacteria, (d) the S-layer (glyco)-proteins are bound to the rigid peptidoglycan-containing layer via secondary cell wall poly-mers. In Gram-negative bacteria, (e) the S-layer is closely associated with the lipopolysaccharide of the outer membrane.
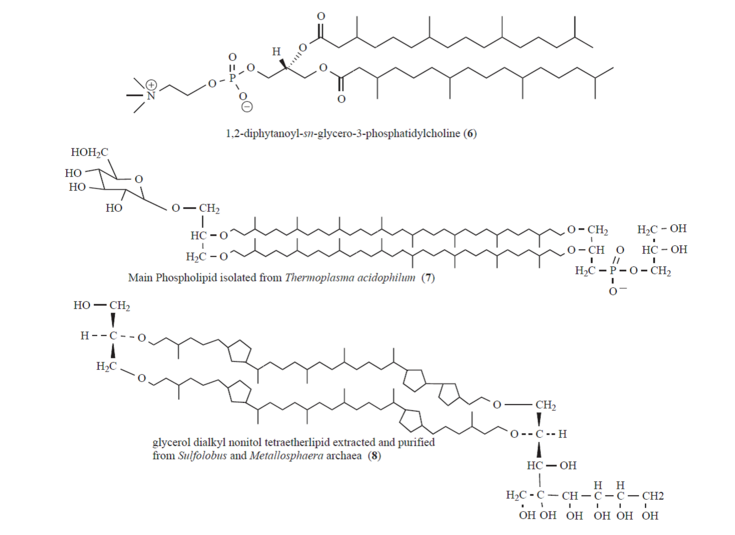
[Translate to English:] Fig. 2: Chemical structures of the phospholipid 1,2-diphytanoyl-sn-glycero-3-phosphatidylcholine (6), the membrane-spanning tetraetherlipids Main Phospholipid (7) isolated from Thermoplasma acidophilum, and glycerol dialkyl nonitol tetraetherlipid (8) extracted and purified from Sulfolobus and Metallosphaera archaea.
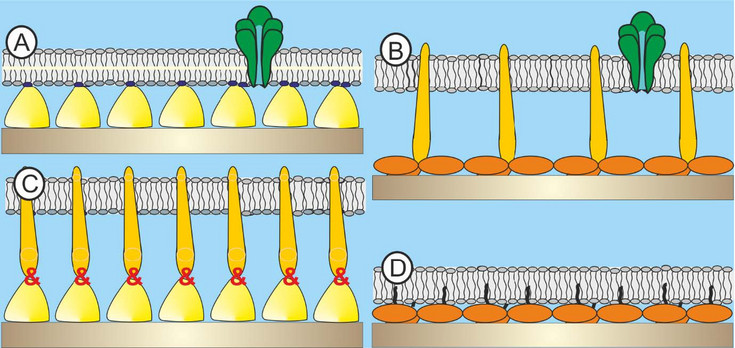
[Translate to English:] Fig. 3: Schematic drawing (not to scale) of A) existing structure: bacterial S-layer protein (SLP)-supported phospholipid bilayer with a membrane protein; B) desired architecture: archaeal SLP-supported core lipids (CL) monolayer with a membrane protein; C) auxiliary hybrid structure: archaeal SLP conjugated to bacterial SLP to form a hydrophobic anchor structure. On this architecture the CL membrane may be generated; D) auxiliary architecture: archaeal SLP modified either by Nature or chemical procedures in order to act as anchoring scaffold for the CL monolayer.
