SCHÜLLER Lab
Fungal Environmental Genetics
Overview
Human fungal pathogens, Stress response, Gene expression, Phenotype profiling, Antifungal drugs, Microbial interactions
Leader: Priv.-Doz. Dr. Christoph Schüller
Research Focus
Environmental adaptations in yeast and human pathogenic yeasts
How do fungal cells adapt to changing environmental conditions like nutrient limitation, different host niches or antifungal treatment? Environmental parameters are constantly sensed by cells. We study specific aspects of cellular stress response in yeast and related human fungal pathogens. For example, cells rapidly adjust their gene expression program according to the internal and external environment. Changing of external physical parameters such as high temperature or osmotic stress elicit immediate activation of signaling pathways leading to changes of enzyme activities and also large scale changes of gene expression patterns. Many factors such as sensors, signaling pathways, dedicated transcription factors, and chromatin structure contribute to these adaptations. For human fungal pathogens like Candida spp. these factors play an important role in adaptation to the host niche and are essential for persistence in the host. Candida species cause mucosal as well as disseminated infections in humans. They can cause life-threatening bloodstream infections especially in immuno-compromised patients, such as neonates and patients undergoing prolonged hospitalization. Furthermore, a number of Candida spp. are associated with episodes of vulvovaginal candidiasis, causing significant discomfort and inconvenience for the afflicted. There is need to elucidate the molecular mechanisms mediating adaptation to the environment to help us to understand why some Candida species are successful and persistent human commensals and to develop new strategies for effective antifungal therapy. We explore phenotypic flexibility and measure phenotypic variation in collections of Candia clinical isolates using high throughput liquid handling.
Current Projects
-
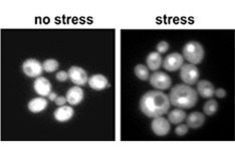
Nuclear localization of Hog1-GFP in response to stress in C. glabrata.
Stress signalling in Candida glabrata
How is signalling of environmental cues wired in the human fungal pathogen Candida glabrata? This yeast is closely related to the perhaps best investigated organism S. cerevisiae. However, the contact with the host provides a number of different and stressful environments for the pathogen.
The clue to pathogenicity might be in specially tuned gene regulation together with a set of adhesin genes and increased thermo- and starvation tolerance.
We suggest that C. glabrata has shifted its metabolic and environmental responses to host environments. In previous work we investigated Stress and starvation responses such as autophagy and their contribution to virulence.
In addition, we are currently analysing the role of the MAPK of the High Osmolarity Glycerol (HOG) pathway, CgHog1. In yeast, this pathway is activated in response to osmotic stress caused by e.g. high extracellular salt or sugar concentrations. In several fungal pathogens, the HOG pathway is at the crossroads of many traits involved in host-pathogen interactions. In clinical Candida isolates a widely differing spectrum of Hog1 activation and stress response rates can be observed and we study the effect of Hog1 activation on the phenotypic level.
-

C. parapsilosis and C. glabrata.
High throughput phenotypic mapping of clinical Candida strains
Fugal pathogens are able to rapidly adapt to a variety of environmental situations. To explore how this flexibility is possible, we investigate the phenotypic variation of a collection of several hundred Candia clinical isolates collected by the Vienna General Hospital during the last decade. Using high-throughput liquid handling robotics (www.bimm-research.at) we determine phenotypes.
The collection is scored for phenotypes such as carbon source utilization, resistance to pH variations, organic acid stress, osmotic stress, antifungals resistance and more. We further investigate the stability of some phenotypes and we try to manipulate selected phenotypes.
Candida-Bacterial microfloral interactions
In the vaginal tract Candida cells compete with the commensal bacterial microflora (e.g. Lactobacillus spp.). We are investigating the interactions that occur between Candida spp. and Lactobacillus species found in the vaginal tract by genome-wide and genetic analysis strategies.
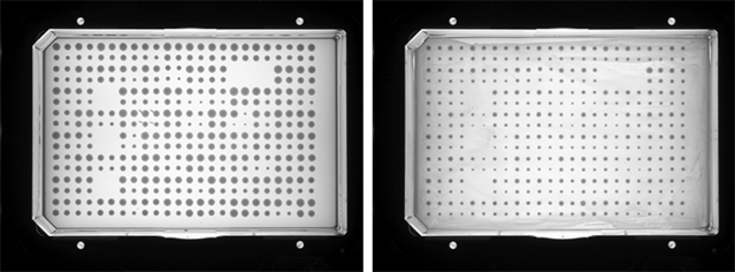
Growth of Candida in the absence (left) and presence (right) of Lactobacillus.
Antifungal peptides effective against human fungal pathogens
To break new ground for development of specific antifungals we aim at the identification of compounds affecting the survival of C. glabrata and related fungi. To this end we are in the process of establishing a genetic screen for antifungal peptides generated by yeast as a synthesis and test system. Using yeast, growth inhibiting or lethal peptides are determined by systematic genetic analysis of the mode of action. Peptides targeting fungal specific processes will be developed further and their function will be addressed by expressing these peptides in C. glabrata and possibly other genetically accessible fungi like A. gossypii and A. nidulans.
Publications
Selected publications:
Weinberger S, Beyer R, Schüller C, Strauss J, Pellis A, Ribitsch D, Guebitz GM. High Throughput Screening for New Fungal Polyester Hydrolyzing Enzymes. Front Microbiol. 2020 Apr 4;11:554. doi: 10.3389/fmicb.2020.00554. PMID: 32390956; PMCID: PMC7193820.
Zangl I, Pap IJ, Aspöck C, Schüller C. The role of Lactobacillus species in the control of Candida via biotrophic interactions. Microb Cell. 2019 Nov 25;7(1):1-14. doi: 10.15698/mic2020.01.702. PMID: 31921929; PMCID: PMC6946018.
Beyer R, Spettel K, Zeller I, Lass-Flörl C, Achleitner D, Krause R, Apfalter P, Buzina W, Strauss J, Gregori C, Schüller C, Willinger B. Antifungal susceptibility of yeast bloodstream isolates collected during a 10-year period in Austria. Mycoses. 2019 Apr;62(4):357-367. doi: 10.1111/myc.12892. Epub 2019 Feb 20. PMID: 30636016.
A constitutive active allele of the transcription factor Msn2 mimicking low PKA activity dictates metabolic remodeling in yeast. Pfanzagl V, Görner W, Radolf M, Parich A, Schuhmacher R, Strauss J, Reiter W, Schüller C. Mol Biol Cell. 2018 Sep 26:mbcE18060389. doi: 10.1091/mbc.E18-06-0389. [Epub ahead of print] PMID: 30256697
Competition of Candida glabrata against Lactobacillus is Hog1 dependent. Beyer R, Jandric Z, Zutz C, Gregori C, Willinger B, Jacobsen ID, Kovarik P, Strauss J, Schüller C. Cell Microbiol. 2018 Aug 15:e12943. doi: 10.1111/cmi.12943. [Epub ahead of print] PMID: 30112857
Klopf E, Schmidt HA, Clauder-Münster S, Steinmetz LM, Schüller C. INO80 represses osmostress induced gene expression by resetting promoter proximal nucleosomes. Nucleic Acids Res. 2017 Apr 20;45(7):3752-3766. doi: 10.1093/nar/gkw1292. PubMed PMID: 28025392; PubMed Central PMCID: PMC5397147.
Kielbassa AM, Ulrich I, Werth VD, Schüller C, Frank W, Schmidl R. External and internal resin infiltration of natural proximal subsurface caries lesions: A valuable enhancement of the internal tunnel restoration. Quintessence Int. 2017;48(5):357-368. doi: 10.3290/j.qi.a37799. PubMed PMID: 28294198.
Kugler KG, Jandric Z, Beyer R, Klopf E, Glaser W, Lemmens M, Shams M, Mayer K, Adam G, Schüller C. Ribosome quality control is a central protection mechanism for yeast exposed to deoxynivalenol and trichothecin. BMC Genomics. 2016 Jun 1;17:417. doi: 10.1186/s12864-016-2718-y. PubMed PMID: 27245696; PubMed Central PMCID: PMC4888481.
Hosiner D, Gerber S, Lichtenberg-Fraté H, Glaser W, Schüller C, Klipp E. Impact of acute metal stress in Saccharomyces cerevisiae. PLoS One. 2014 Jan 9;9(1):e83330. doi: 10.1371/journal.pone.0083330. eCollection 2014. PubMed PMID: 24416162; PubMed Central PMCID: PMC3886979.
Jandric Z, Gregori C, Klopf E, Radolf M, Schüller C. Sorbic acid stress activates the Candida glabrata high osmolarity glycerol MAP kinase pathway. Front Microbiol. 2013 Nov 26;4:350. doi: 10.3389/fmicb.2013.00350. eCollection 2013. PubMed PMID: 24324463; PubMed Central PMCID: PMC3840799.
Reiter W, Klopf E, De Wever V, Anrather D, Petryshyn A, Roetzer A, Niederacher G, Roitinger E, Dohnal I, Görner W, Mechtler K, Brocard C, Schüller C, Ammerer G. Yeast protein phosphatase 2A-Cdc55 regulates the transcriptional response to hyperosmolarity stress by regulating Msn2 and Msn4 chromatin recruitment. Mol Cell Biol. 2013 Mar;33(5):1057-72. doi: 10.1128/MCB.00834-12. Epub 2012 Dec 28. PubMed PMID: 23275436; PubMed Central PMCID: PMC3623084.
Jandric Z, Schüller C. Stress response in Candida glabrata: pieces of a fragmented picture. Future Microbiol. 2011 Dec;6(12):1475-84. doi: 10.2217/fmb.11.131. Review. PubMed PMID: 22122443.
Roetzer A, Klopf E, Gratz N, Marcet-Houben M, Hiller E, Rupp S, Gabaldón T, Kovarik P, Schüller C. Regulation of Candida glabrata oxidative stress resistance is adapted to host environment. FEBS Lett. 2011 Jan 21;585(2):319-27. doi: 10.1016/j.febslet.2010.12.006. Epub 2010 Dec 13. PubMed PMID: 21156173; PubMed Central PMCID: PMC3022126.
Roetzer A, Gabaldón T, Schüller C. From Saccharomyces cerevisiae to Candida glabratain a few easy steps: important adaptations for an opportunistic pathogen. FEMS Microbiol Lett. 2011 Jan;314(1):1-9. doi: 10.1111/j.1574-6968.2010.02102.x. Epub 2010 Sep 16. Review. PubMed PMID: 20846362; PubMed Central PMCID: PMC3015064.
Batova M, Klobucnikova V, Oblasova Z, Gregan J, Zahradnik P, Hapala I, Subik J, Schüller C. Chemogenomic and transcriptome analysis identifies mode of action of the chemosensitizing agent CTBT (7-chlorotetrazolo[5,1-c]benzo[1,2,4]triazine). BMC Genomics. 2010 Mar 4;11:153. doi: 10.1186/1471-2164-11-153. PubMed PMID: 20202201; PubMed Central PMCID: PMC2841119.
Roetzer A, Gratz N, Kovarik P, Schüller C. Autophagy supports Candida glabrata survival during phagocytosis. Cell Microbiol. 2010 Feb;12(2):199-216. doi: 10.1111/j.1462-5822.2009.01391.x. Epub 2009 Oct 6. PubMed PMID: 19811500; PubMed Central PMCID: PMC2816358.
Klopf E, Paskova L, Solé C, Mas G, Petryshyn A, Posas F, Wintersberger U, Ammerer G, Schüller C. Cooperation between the INO80 complex and histone chaperones determines adaptation of stress gene transcription in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 2009 Sep;29(18):4994-5007. doi: 10.1128/MCB.01858-08. Epub 2009 Jul 20. PubMed PMID: 19620280; PubMed Central PMCID: PMC2738301.
Funding Agencies
Collaborations
- Birgit Willinger (MUW)
- Gerhard Adam (DAGZ, BOKU)
- Gustav Ammerer (MFPL, Univ. of Vienna)
- Pavel Kovarik (MFPL, Univ. of Vienna)
- Toni Gabaldón (CRG, Barcelona)
- David Cánovas (US, Seville)


