News from lignin chemistry: first quantitatively approximated lignin structure published
One of the world's largest databases on technical lignins (i.e. lignins obtained by biomass digestion) and native lignins has been developed for years at the Institute of Chemistry of Renewable Resources, in cooperation with the BOKU Core Facility ALICE ("Analysis of Lignocellulosics"). In contrast to the chemically relatively simple cellulose structure, lignin is an extremely complex macromolecule in which several basic building blocks (monomers) are linked together in many different ways. This structural heterogeneity of native lignins is further enhanced in technical lignins by chemical pulping processes, e.g. in the paper and pulp industry. This is the main reason why it is so difficult to use lignins as a material, non-energy source and to break them down into small, low-molecular structures in a targeted manner, and why lignin is still used almost exclusively for energy production today. However, lignin represents one of the most important carbon sources and starting materials for the biorefineries of the future.
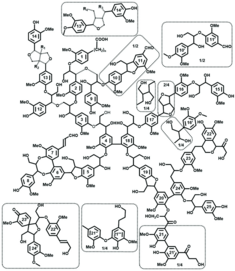
There are probably no two lignin molecules in the world that are completely identical. Although the most important structural elements of lignins are well known, e.g. from nuclear magnetic resonance (NMR) spectroscopy studies, the qualitative structures could not be reconciled with the quantitative analytical data so far: one almost always assumed approximations and only some of many structural elements, which of course made it difficult to develop reactivity concepts and structure-property-application relationships.
Based on decades of chemical-analytical lignin expertise and with the help of the new lignin database, Dr. Irina Sulaeva, Prof. Antje Potthast and Prof. Thomas Rosenau from the Institute of Chemistry of Renewable Resources and their colleagues from Aalto University, Finland, RISE Stockholm and the University of British Columbia at Vancouver, Canada, have now succeeded in developing a lignin structural formula that is also quantitatively meaningful for the first time. Initial responses from the international lignin research community see this as an important milestone in understanding the structure and reactivity of lignins, which ultimately provides a foundation for targeted, non-empirical lignin use.
More information can be found here: https://pubs.rsc.org/en/content/articlelanding/2020/gc/d0gc00926a#!divAbstract