Signal Integration
Gebiet
Gebiet
O-GlcNAcylation, plant development, signal transduction and adaptation
Projektleiterin: Dr. Doris Lucyshyn
Ausrichtung
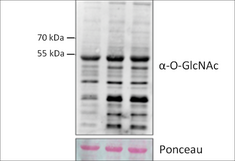
Proteins can be regulated by a multitude of different post-translational modifications (PTMs), conferring complexity to their function and stability. These modifications are essential components of signalling pathways in the course of development, but often also in response to changes in the environment. O-glycosylation of nuclear and cytoplasmic proteins is one of these PTMs. In contrast to other glycosylation events, a single monosaccharide - for example N-acetylglucosamine, (GlcNAc) - is attached to serine or threonine residues of a high number of very diverse proteins.
The O-GlcNAc modification is very well characterized in animals, where it is essential for development and decisively involved in a range of signalling events, often affecting protein interactions and competing with other O-linked PTMs. Animals carry one O-GlcNAc transferase (OGT), and a specific O-GlcNAc hydrolase (OGA), making this modification reversible and highly dynamic. In contrast, plants have two structurally related O-glycosyltransferases, competing for the same targets: the O-GlcNAc transferase SECRET AGENT (SEC) and the protein O-fucosyltransferase SPINDLY (SPY) show high sequence homology, but their activity can have opposite effects on their target proteins. This mechanism of counteracting O-glycosyltransferases seems to be specific for plants. Its importance for plant development is apparent from genetic analysis, as a double knockout of the two enzymes is embryonic lethal. Interestingly, it is not clear if the modifications can also be removed, as specific O-GlcNAc or O-fucose hydrolases have not been identified as yet.
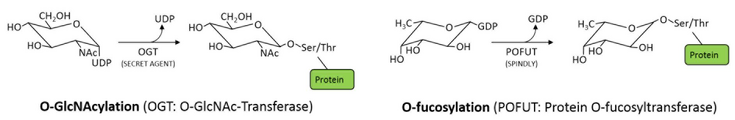
The molecular function of these modifications is characterized only for few targets, and so far the techniques to study this type of glycosylation are limited. We are currently working on these aspects of O-glycoslytion and potential fundamental differences between plants and animals in this context, using a combination of different approaches, including identification of targets of SPY and SEC, as well as interacting proteins. Moreover, we are studying the importance of O-glycosylation for specific aspects of plant development and cell biology.