Gasser Lab: Yeast Molecular Biotechnology
Molecular Biotechnology determines and describes the underlying molecular principles of biotechnological production processes. Among these products, recombinant proteins (e.g. biotherapeutics and industrial enzymes) are of paramount importance. To accelerate the development of production strains it is crucial to understand the molecular physiology of the host, and the specific limitations that the product may exert on expression.
The yeast Pichia pastoris (syn. Komagataella spp) has emerged as an efficient host for recombinant protein production. We focus on the comprehensive understanding of protein folding and secretion, and on cell engineering for the improvement of these processes (BioTop Project 2019; BioTop Project 2015). Furthermore, we are part of the MSCA-ITN SECRETERS which designs new-generation microbial platforms for the production of disulphide-bonded and 'difficult-to-express' proteins (http://secreters-msca-itn.eu/).
In the “CD-Laboratory for growth-decoupled protein production in yeast”, the connection between cell growth and protein secretion is analyzed by looking at yeast cells cultivated at very low specific growth rates, using the yeast P. pastoris as a model system (link).
Systems biology analyses include genome, transcriptome, proteome, metabolome, and fluxome analyses, and metabolic modelling, these high throughput technologies are performed in cooperation with the Department of Chemistry, FH Campus Wien Bioinformatics group, and VBCF. Computational metabolic modelling is performed in joint projects with Metabolic Modelling group. Curated genome sequences of different P. pastoris strains are available at www.pichiagenome.org.
Pichia Genome Database
This database hosts up-to-date annotation information of the genome of the methylotrophic yeast Pichia pastoris (Komagataella spp.), containing the new manually achieved annotation of K. phaffii strain CBS7435, and basic sequence and annotation information taken from the GenBank data of various Komagataella strains and species.
Additionally the sequence information of S. cerevisiae strain S288c is also available for comparison and homolog display.
Latest Publication
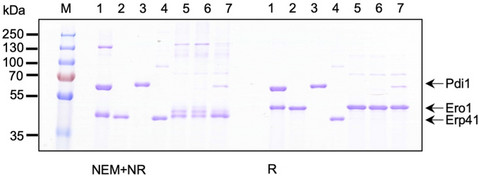
Komagataella phaffii Erp41 is a protein disulfide isomerase with unprecedented disulfide bond catalyzing activity when coupled to glutathione
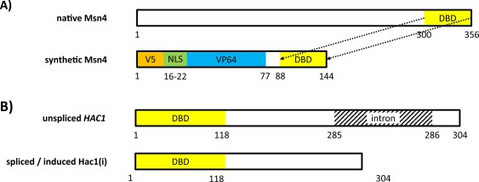
Synthetic activation of yeast stress response improves secretion of recombinant proteins

BOKU Töchtertag 2022
Workshop: Lebende Proteinfabriken: von der Zelle zum Produkt

Protein production dynamics and physiological adaptation of recombinant Komagataella phaffii at near-zero growth rates.

Systematic sequence engineering enhances the induction strength of the glucose-regulated GTH1 promoter of Komagataella phaffii
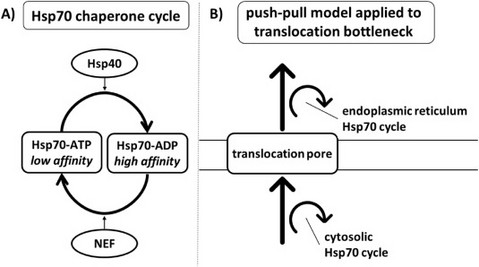
Pushing and pulling proteins into the yeast secretory pathway enhances recombinant protein secretion
BOKU Erfinderin 2019
Brigitte Gasser wurde 2019 als eine der BOKU Erfinderinnen 2019 gewürdigt.
In diesem Video stellt sie sich selbst und ihre Forschungsarbeit vor.

Characterising the metabolic rewiring of extremely slow growing Komagataella phaffii.
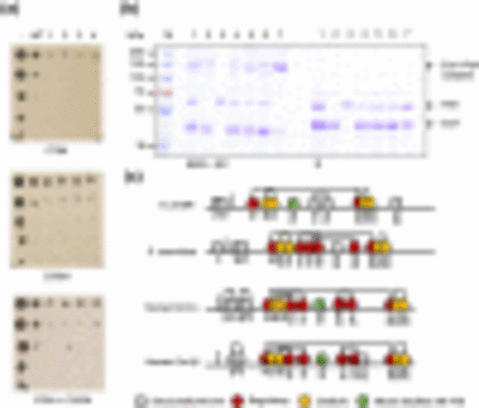
Biochemical analysis of Komagataella phaffii oxidative folding proposes novel regulatory mechanisms of disulfide bond formation in yeast
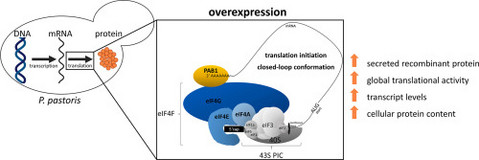
Going beyond the limit: Increasing global translation activity leads to increased productivity of recombinant secreted proteins in Pichia pastoris.
FEMS Yeast Research Best Article Award 2021
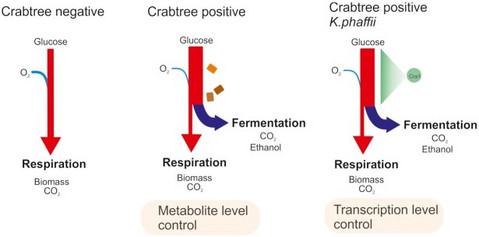
What makes Komagataella phaffii non-conventional?
Established tools and emerging trends for the production of recombinant proteins and metabolites in Pichia pastoris.
Brigitte Gasser, Assoc. Prof. Priv.Doz.DI Dr.nat.techn.
- Stellvertreterin
- Institut für Mikrobiologie und Mikrobielle Biotechnologie
- brigitte.gasser@boku.ac.at
- Telefon
- +43 1 47654-79033
- Fax
- +43 1 47654-79009
- Postadresse
-
Mikrobiologie und Mikrobielle Biotechnologie
Muthgasse 18/V
1190 Wien - Büro
-
MUG1-04/03
Muthgasse 18/IV
1190 Wien