Molecular Plant Physiology and Crop Biotechnology
Our research interests
Using cell biology approaches to understand intracellular protein trafficking and deposition in specialized cell types, with special focus on crop plants. Following the morphological dynamics of the endomembrane system in developing seed storage tissues. Monitoring ER-stress and protein aggregation in crop and model seeds.
Bioencapsulation of recombinant proteins in plant storage organelles. Design of novel storage organelles for special applications. Production of pharmaceutical proteins in seed crops.
Application of new plant breeding technologies, in particular gene editing, to improve the performance of host plants for the production of recombinant proteins.

Seeds come in all shapes and sizes
- Seeds and other plant tissues: cell biology
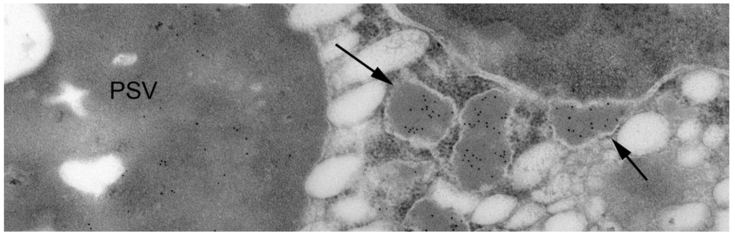
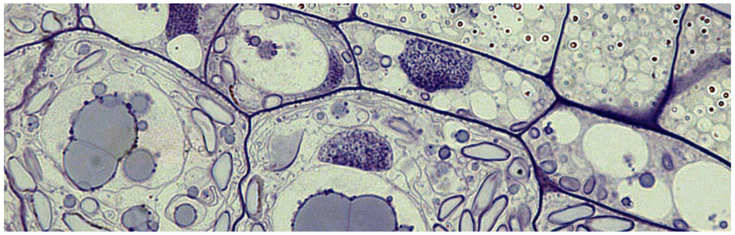
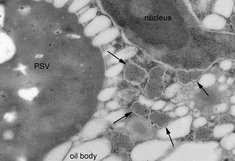
Seeds have the ability to store large amounts of proteins as a source of nitrogen for the seedling. The endomembrane system of seed storage tissues is therefore dedicated to protein synthesis, and specialized organelles are formed for the accumulation of storage proteins, which are ultimately deposited either within protein bodies derived from the endoplasmic reticulum, or in protein storage vacuoles (PSVs). Protein transport occurs via developmentally regulated trafficking routes, and the endomembrane system undergoes extensive reorganization and rearrangement.
To gain further insight into these dynamic membrane reorganization events in the developing seed we are using a combination of advanced imaging approaches including live cell imaging, transmission electron microscopy (TEM), scanning electron microscopy (SEM), immunoelectron microscopy and volume electron microscopy such as electron tomography and serial block face scanning electron microscopy.

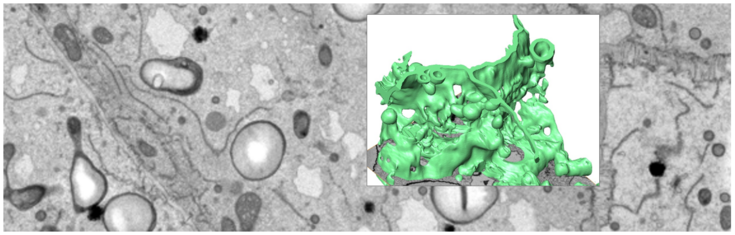
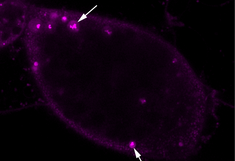
Rendering of ER membranes in developing maize seed. Serial Block Face-SEM.
- Monitoring and modulating ER-stress and protein aggregation in seeds
Cells need to react to abiotic and biotic disturbances that affect ER protein load, folding or traffic. As a consequence, cells may either be able to revert to their normal state or to adapt their cellular organization to the new conditions, or they may undergo apoptosis. For stress detection, the ER membrane and its surrounding are populated with sensors detecting imbalances in protein synthesis and folding that in turn activate the unfolded protein response (UPR) or ER stress response.
Recombinant protein production targeted to the secretory pathway may constitute such a disturbance as the process burdens the ER of the host cell with extra workload for protein folding, quality control and trafficking. We are particularly interested in the ER stress response in seeds upon recombinant protein production. Seed tissues have evolved to cope with expression and storage of a demanding protein load, and therefore these tissues offer a special opportunity for recombinant protein expression and accumulation. We aim to obtain a better understanding of the stress response in seeds and to remodel plant seed and non-seed tissues as efficient hosts for recombinant protein production.
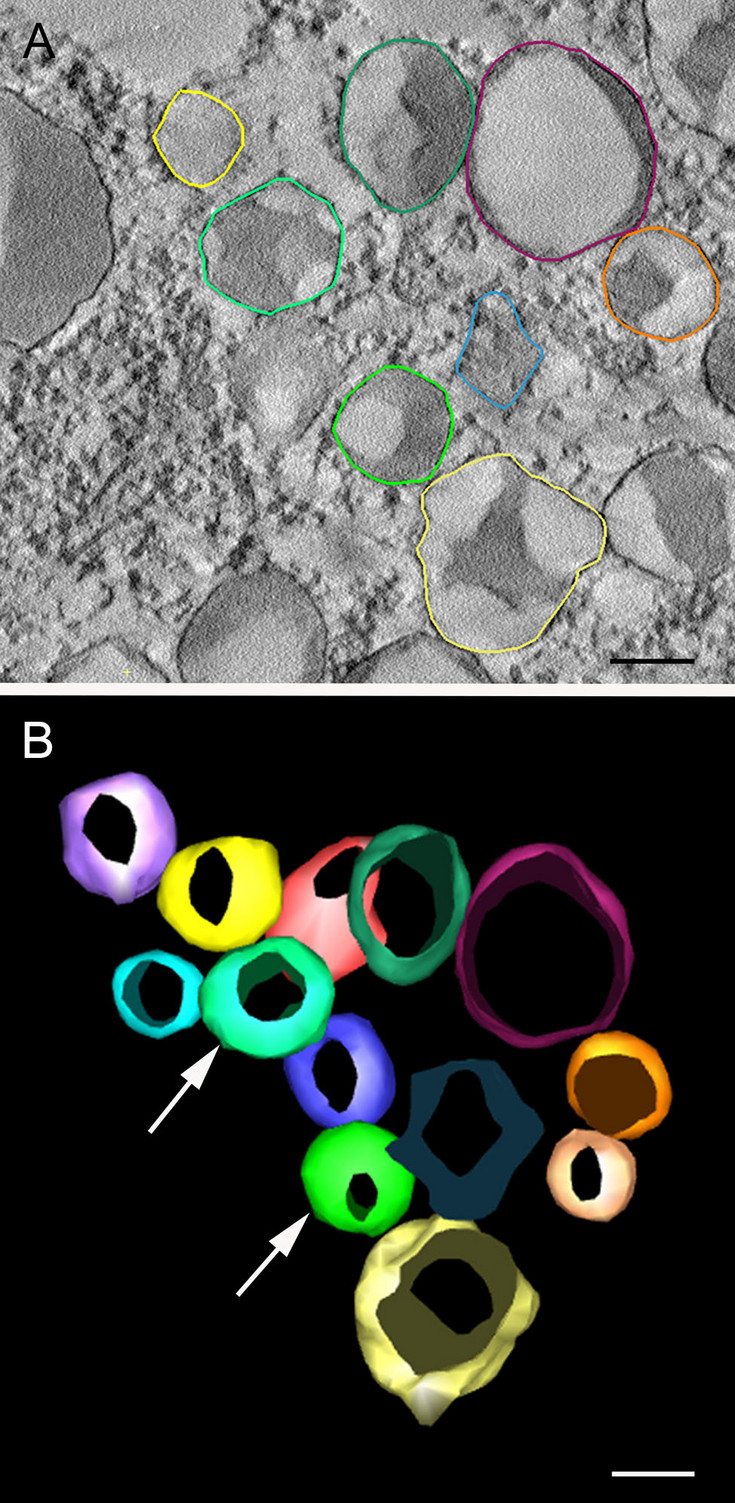
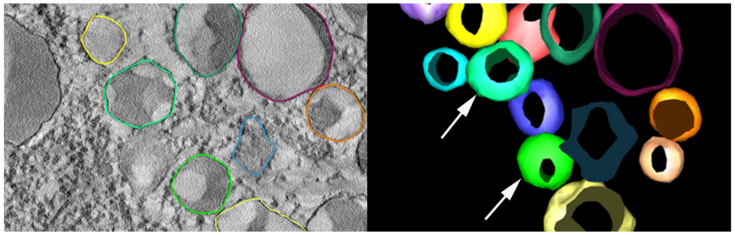
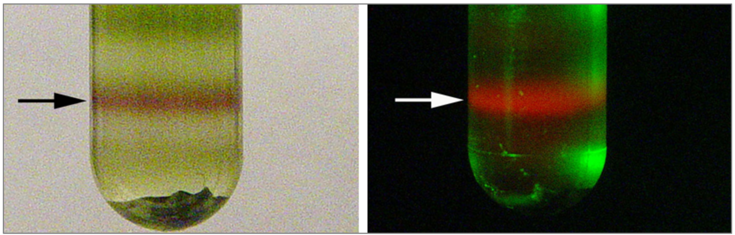
Russell bodies induced by recombinant proteins in Arabidopsis cotyledons. A. Serial section used for modelling, B. 3D model. Electron tomography (Arcalis et al. 2019)
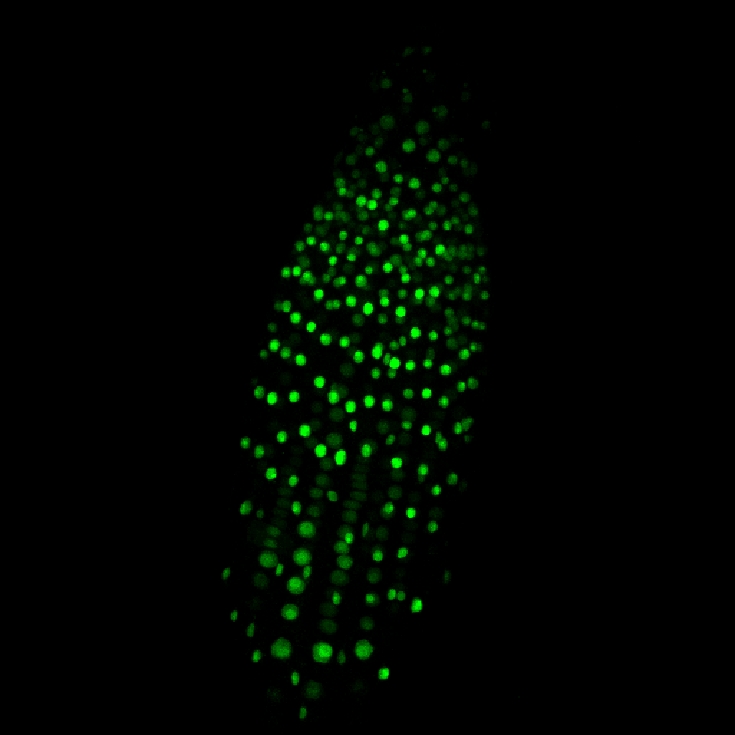
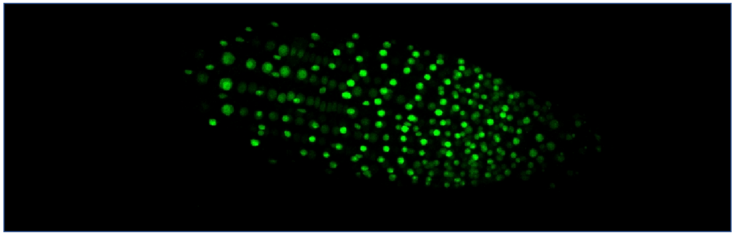
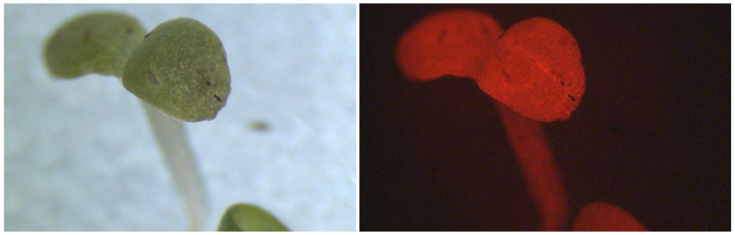
Recombinant fluorescent ER stress sensor. Arabidopsis root tip. CLSM.
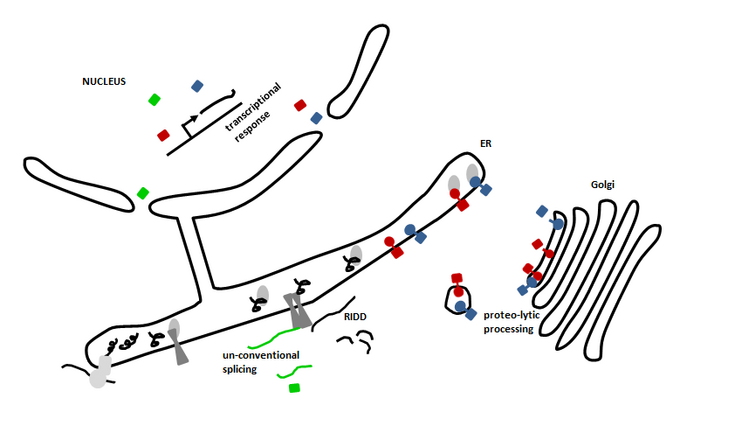
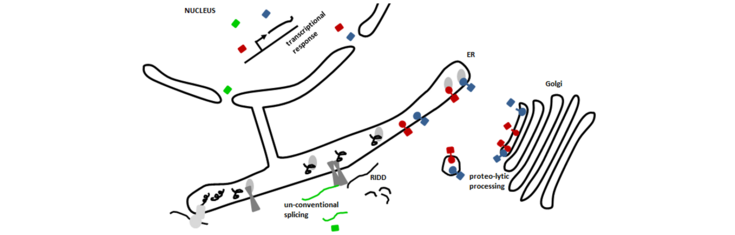
Unfolded protein response (UPR) in plants
Un-conventional splicing of bZIP60 (green) by IRE1 (dark grey) and proteolytic processing of bZIP28 and bZIP17 (red and blue) leads to mobile transcription factors that are able to enter the nucleus. There they establish a transcriptional response to supply the ER with factors to alleviate endoplasmic reticulum (ER) stress, e. g. chaperones. In order to reduce the overall translational load regulated IRE1-dependent decay (RIDD) cleaves mRNAs encoding for secretory proteins. These processes are conserved ER stress mechanisms in eukaryotes.
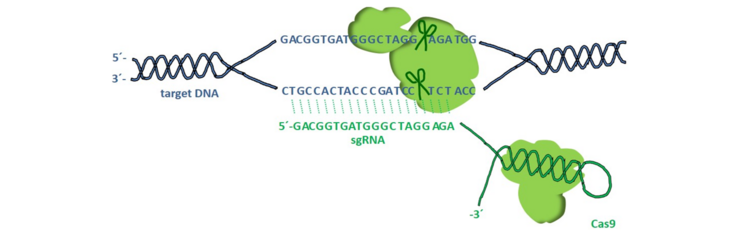
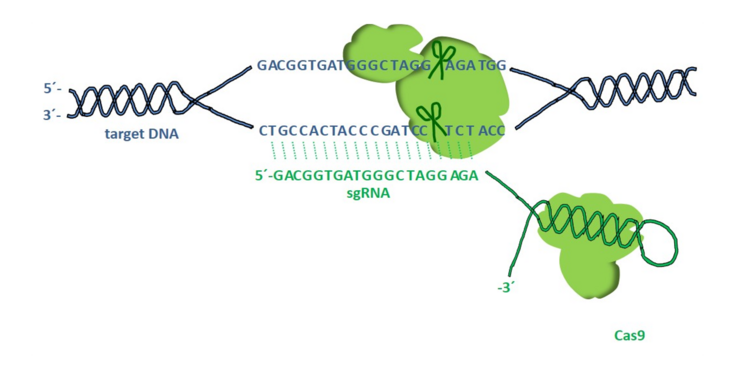
- Genome editing in crop plants
Targeted genome editing with the CRISPR/Cas9 system is a convenient tool for introducing mutations in crop plants. One example is barley in which genomic fragment deletions have been produced in the endo-N-acetyl-β-d-glucosaminidase (ENGase) gene to alter glycoprotein modifications in cereal seeds. Additional target gene knockouts in barley and Nicotiana species include regulators of endoplasmic reticulum (ER) stress and metabolic pathways to improve the suitability and performance of plants for the production of pharmaceutically and industrially valuable compounds.
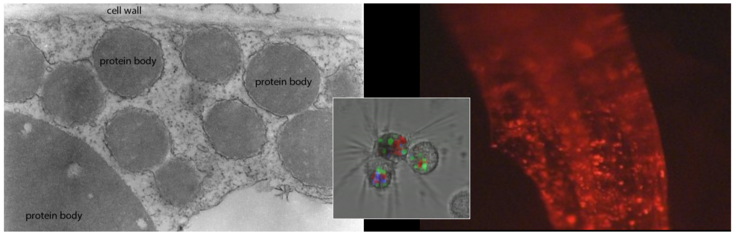
- Bioencapsulation
Naturally occurring protein bodies are the storage sites for endogenous proteins in cereal seeds and offer an appealing strategy for the in vivo microencapsulation of recombinant proteins for mucosal application. In addition, the induction of ectopic protein storage organelles in leaf tissues can be exploited for the encapsulation of pharmaceutical proteins into microparticles, which are readily taken up by mammalian target cells. De novo induction allows us the design of multi-component protein bodies consisting of several protein layers with different resistance to degradation in the digestive system, and these may serve as a valuable tool not only for edible vaccines but also as a slow release drug delivery system for other pharmaceuticals.
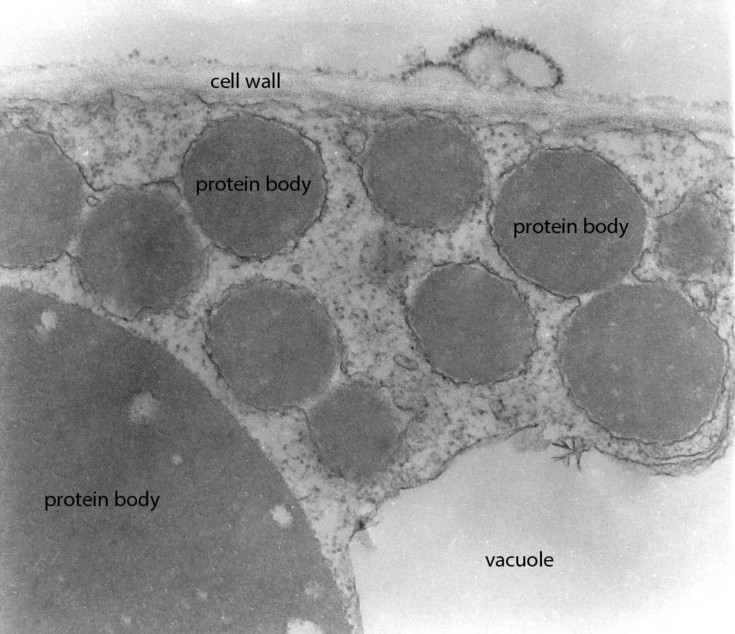
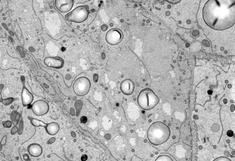
Ectopic protein bodies expressed in N. tabacum leaf (TEM)