Introduction
Within the Institute for Biophysics, the understanding of self-assembly and hierarchical supramolecular structures is one of our major interest. For this purpose, we put a special emphasis on the organic/non-organic interface utilizing a bottom-up approach.
By taking benefit of the possibilities to modify surfaces both chemically and physically new biomimetic interfaces can be designed and produced. Such interfaces can have 2D (i.e. supported lipid bilayers or protein layers) or 3D (i.e. lipid vesicles, polymer capsules) structure.
The control of the roughness, wetting, electrical, chemical, and antifouling properties of such interfaces leads to a wide application range, i.e. biosensing, molecular release, antifouling, scaffolds for cell attachment, and crystal growth (mineralization). Thus the functional films are composed of diverse molecules, such as lipids, proteins, peptides, and various synthetic polymers.
Methods
Coatings and hierarchical structures are made by layer-by-layer deposition, dip-coating, spin-coating, vapor deposition, ATR polymerization, and lithographic approaches.
Different experimental techniques are used to monitor as function of time and to characterize the newly form interfaces and hierarchical structures:
Chemical modifications
One of the most extended methodologies to generate new interfaces is based on the direct attachment (adsorption) of small molecules or polymers to the substrate of interest either in the shape of ”monolayers” (self-assembled monolayers) or transferred with the help of a soft stamp (micro-contact printing). The latter enables restraining the location of these molecules to the particular geometrical pattern defined by a stamp, while the former ensures a full coverage of the support. This can be done by means of dip-coating or spin-coating, attending to the density of the solution, or by vapor deposition, under a saturated atmosphere of the compound, when dissolved in highly volatile solvents. In this way, the surface properties can be completely tuned at nanometer scale as required.
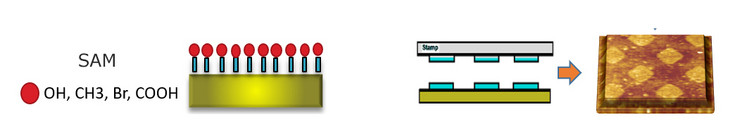
Left: Self-assembled monolayers on glass, silicon dioxide or gold substrates. Covalent chemical modification involves the use of either silanes or thiols in which the end-free hydrophobic chain has a tunable length and an active group that determines the hydrophobic and electrical properties. Such chemical groups can also initiate polymerization reactions. Layer by layer can be used to physically adsorbed biopolymers.
Surface-supported lipid bilayers & (bio)polymeric coatings
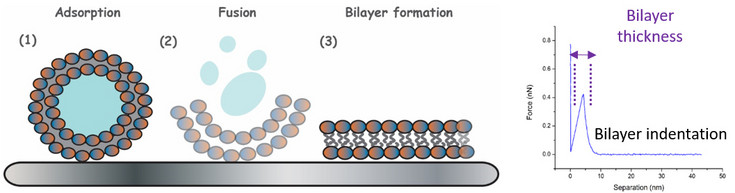
The use of artificial (biomimetic) membranes as models for biological systems is much extended in materials science when willing to test in vitro their interaction with other systems (i.e. proteins, micro- and nano-particles, or drugs). Indeed, by tuning the composition of (phospho)lipids and cholesterol (in the form of vesicle and later bilayer) one can mimic the structure and behavior of different cell membranes (i.e. mosquito larvae, mammalian cells, etc.). A similar concept applies to other bioactive coatings (i.e. polyelectrolytes, fibronectin,) that share their prospective use as interface for the binding of additional functional elements or the attachment of cells.
Protein crystal layers
Proteins can act as building blocks for the construction of thin biomimetic films. There are particular examples in which self-assembly of such individual molecules leads to the formation of a regular crystal-like lattice through self-assembly. Depending on the number of identical protein subunits forming the repetition unit in the lattice, oblique (p1, p2), square (p4), or hexagonal (p3, p6) lattice symmetries can be identified, with cell dimensions of only a few nanometers and a constrained central pore acting as communication channel between both sides of the film. Up to now we have worked with bacterial S-layer proteins (SbpA, SbsB) and Annexin V. These proteins, when “isolated” in solution, have the ability to form organized structures by self-assembly. The properties of the final crystalline arrays can be controlled by varying the interaction protein-protein and protein-substrate. This is achieved by selecting the appropriate physico-chemical parameters (concentration, ionic strength, hydrophobicity, etc.). However, the self-assembly process itself is not totally understood remaining many open questions.
Among the different properties of the bacterial protein layers we can highlight non-fouling activity (when the crystalline structure is intact), and its use in drug release systems (through the nano-pores of the crystalline structure).
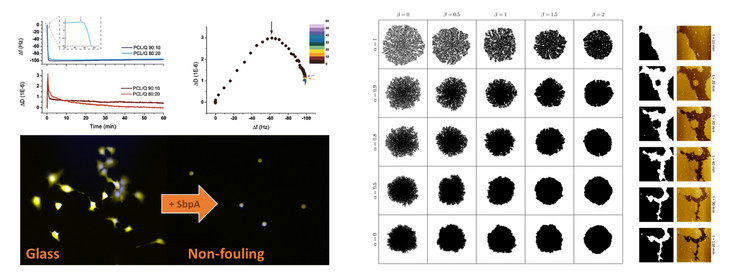
Left above: Monitoring with quartz crystal microbalance with dissipation of the adsorption of bacterial proteins and protein layer formation on silicon wafers.
Left below: application of the antifouling properties of the crystalline protein layers. Cells attach on glass but not on the protein layer.
Cell & particle attachment
Most of the coatings and laser induced micro- and nanostructures produced at the institute and by our collaborators share a final biocompatibility goal, with focus on optimal cell attachment and proliferation. Cell attachment is usually attempted either via coatings containing extra-cellular matrix (ECM) proteins that are recognized by transmembrane receptors (i.e. fibronectin), or via interactions of different nature (i.e. electrostatic forces). Based on the substrate affinity cells could experience morphological changes. In such regard, novel (bio)polymer-based alternatives can be taken in mind for the development of cell-friendly surfaces, which broaden the spectrum from usually employed fibronectin, collagen and laminin coatings offering anchoring attachment via RGD and IKVAV aminoacid moieties. For a hybrid synthetic-natural interface, polyelectrolytes, polylactide, polydimethyl siloxane (PDMS), and mucin films offer interesting possibilities, since their physical and chemical properties are easy to tune.
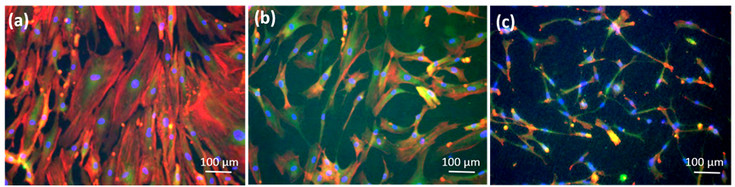
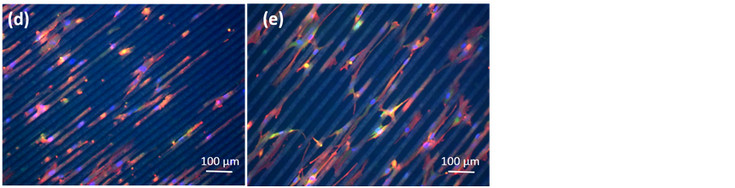
Immunofluorescence images of mesenchymal stem cells (MSCs) grown on cover slips or on 4 different Poly-L-Lactide (PLLA) surfaces after 14 days in culture: (a) MSCs on cover slips; (b) MSCs on flat PLLA; (c) MSCs on rough PLLA; (d) MSCs on groves of interspacing of 15 µm; (e) MSCs on grooves of interspacing of 25 µm.
Focal adhesions marked with Vinculin are shown in green, cell cytoplasm marked with Phalloidin in red and cell nuclei marked with DAPI in blue.