Research
Engineering non-antibody-based binding domains

One focus of our group is the development and application of non-antibody-based binding domains. These small, stable and well-expressed binders offer many critical advantages for a wide range of applications. Depending on the needs for the specific application, we have two main options available in our laboratory:
“Reduced charge Sso7d” (rcSso7d)-based binders are distinguished by their exceptionally high stability, which is a result of their hyperthermostable parental scaffold protein (Tm of 96°C). As a result, these binders are typically well-expressed in a range of expression hosts and highly resistant to aggregation. However, the non-human origin poses some risk of immunogenicity when used for therapeutic applications.
Therefore, we have also developed a binder scaffold platform based on human protein domains. More specifically, we developed a novel engineering platform for the generation of antigen binding domains that are specifically tailored to CAR T cell applications. For that purpose, we initially defined a “wish list” of properties of an ideal CAR antigen binding domain (human origin, small size, high stability, efficient expression on human T cells, no aggregation/clustering, among others). To systematically identify the best suited scaffold candidates, we screened the entire Protein Data Bank (PDB) in silico (>150,000 structures), followed by a comprehensive set of wet lab experiments. This finally yielded two binder scaffolds with perfect features for CAR T applications. These “minimalistic CAR binding domains” (miniCARbids) can be engineered with antibody-like affinities (nM to pM range) against virtually any antigen of interest and they show high potency in human CAR T cells.
Engineering drug-regulated protein switches
Another priority in our group is the generation of drug-regulated protein switches, i.e. protein domains that interact with each other in a drug-dependent manner.
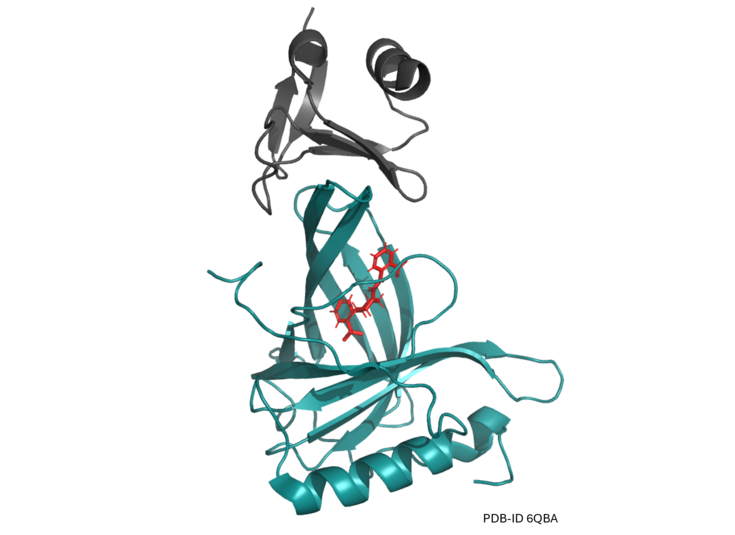
One of our switch systems is based on human retinol binding protein 4 (hRBP4). hRBP4 undergoes a conformational change upon binding to the synthetic drug A1120. Using protein engineering technologies, we developed binders that specifically recognize hRBP4 in the drug-loaded state, thus yielding a protein-protein interaction that can be “turned on” via administration of the drug. The picture above shows the engineered binder RS3 in complex with A1120-loaded hRBP4.
In another switch system, we re-engineered caffeine-specific nanobodies to yield a heterodimeric, caffeine-regulated switch. That is, these heterodimers are inactive in the absence of caffeine, but potently activated upon addition of this small molecule.
Both switch systems (hRBP4/A1120-based and caffeine-responsive nanobodies) enable precise control of CAR T cell function both in vitro and in vivo.
Targeting activated receptor conformations
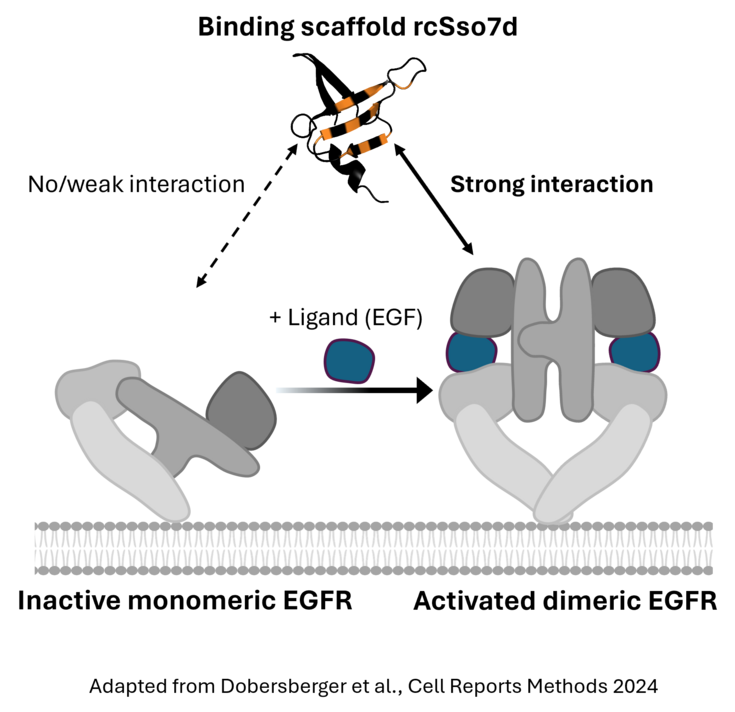
Many cancers have evolved mechanisms to constitutively activate surface receptors such as EGFR. To improve the tumor specificity of cancer therapies such as CAR T cells, we have developed an engineering strategy to specifically target those activated receptor states. Our engineered binders show high affinity to activated EGFR, but no detectable interaction with the inactive conformational state. We are currently exploring these engineered binding domains in the context of CAR T cells to yield potent yet tumor-specific CAR T cells.
