Activation of pattern recognition receptors with carbohydrae-based biomolecules as immunotherapeutic tool for cancer
Innate immunity-related factors play an important role in the development of various malignancies, with several components being explored as novel therapeutic targets or adjuvants. Cancer immunotherapy using immune checkpoint inhibitors has emerged as a major breakthrough, offering the potential for cure even in advanced stages for certain cancer types and patient subgroups. Beyond enhancing adaptive immunity, innate immunity is increasingly becoming a focus in cancer immunotherapy development. Modulation of the innate immune response can be achieved by directly targeting immune cells within the tumor microenvironment with small molecules that engage host innate immune receptors and trigger protective pro-inflammatory responses.
We are developing carbohydrate-based biomolecules as agonists for various pattern-recognition receptors (PRRs) of the innate immune system to investigate the effects of NF-κB-mediated local and systemic inflammation on tumor growth and to advance immune adjuvant therapies.
Selected contributions
Nature Communications (2025), 16, 4164
Cryo-EM structures of picomolar TLR4 agonists:
https://www.rcsb.org/structure/8WQT
https://www.rcsb.org/structure/8WTA
https://www.rcsb.org/structure/8WRY
https://www.rcsb.org/structure/8WO1
https://www.rcsb.org/structure/8WSA
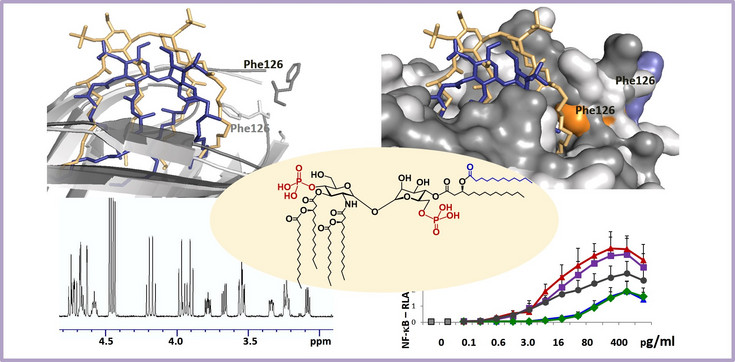
Synthetic Endotoxin mimetics as tools for studying LPS-protein interactions
Toll-like receptor 4 (TLR4) activation is mediated by the binding of lipopolysaccharide (LPS or Endotoxin) to the central hydrophobic pocket of the co-receptor protein myeloid differentiation factor 2 (MD-2), followed by the dimerisation of two ternary ligand-receptor complexes.
LPS from different bacterial species induce different degrees of receptor complex dimerisation, which regulate the subsequent induction of pro-inflammatory signaling. To elucidate the molecular basis of LPS-induced activation of innate immune responses, we design and chemically synthesise unnatural carbohydrate-based LPS mimetics that can interact with LPS-recognising proteins and are perfect tools to study the host-pathogen interaction and the structural basis of ligand recognition by the innate immune receptors.
Our recent findings, based on the immunofunctional studies of synthetic endotoxin mimetics derived from β(1↔1)α and α(1↔1)α linked disaccharide scaffolds, reveal that the 3D molecular shape and ternary structure of the protein-bound diglucosamine backbone of lipid A is one of the most critical determinants of endotoxicity.
We perform synthetic studies on the stereoselective assembly of the non-reducing α,α- β,α- and β,β-1,1'-linked orthogonally protected disaccharides under simultaneous stereocontrol at two anomeric centres. The synthetic disaccharide backbones are then chemically substituted with various functional groups to provide biologically active endotoxin mimetics as tools for immunobiology and valuable probes for studying LPS-protein interactions.
Selected contributions
Angewandte Chemie, Int. Ed. (2024), 63, e202408421.
Pharmaceuticals (2023), 16, 23.
Chemistry (2022), 28, e20220054
Front. Immunol. (2021), 12, 631797
Front. Immunol. (2021), 12, 631797
Chem. Sci. (2018), 16, 3957-3963
ChemMedChem (2018), 13, 2317-2331
J.Med.Chem. (2015), 57, 8056-8071
Innate Immun. (2015); 21, 490-503
ACS Chem.Biol. (2013), 8, 2423-2432