Plant Glycoengineering
Our Research Interests
Cell and Molecular Biology, Plant Molecular Farming, Plant Glycobiology and Glyco-engineering, Modulation of gene expression, Glyco-dependent protein-protein interactions.
Leader: Alexandra Castilho
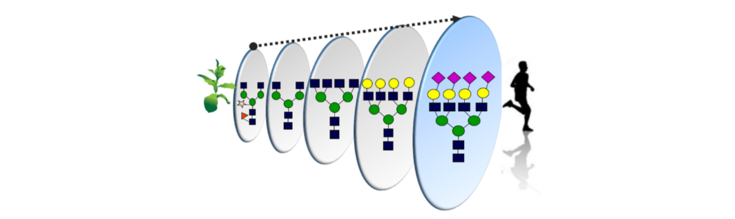
Orientation
Plant Molecular Farming (PMF) refers to the production of valuable proteins through the use of plant biotechnology. Despite great successes, expression of recombinant proteins in plants still presents several bottlenecks. Protein glycosylation is one of the most important post-translational modifications and while N-glycan synthesis in the endoplasmic reticulum (ER) is relatively well conserved in eukaryotes, processing of N-glycans in the Golgi apparatus is kingdom specific and major differences in the glycosylation pathway of plants and animals, hampers the production of relevant glycoproteins in plants. Glyco-design is a powerful tool to optimize glyco-profiles of therapeutic glycoproteins for efficacy. Our research is directed to develop, optimize and evaluate methods to tailor the structures of glycans on relevant recombinant proteins produced in Nicotiana benthamiana, currently the most appropriate host to be used in PMF. We use a variety of approaches for the genetic manipulation of glycosyltransferases (GTs), glycohydrolases (GH), nucleotide sugar interconverting enzymes and transporters to control the glycan biosynthesis in N. benthamiana. The outcome of our research can be applied to identify individual structures fundamental for glycan-mediated processes and facilitated the development of ‘biobetter’ proteins with customized glycan profiles that enhance their activities.
Milestones
N. benthamiana plants are the most suitable platform for the transient expression of recombinant proteins via ‘agroinfiltration’, without the need for genetic transformation and can tolerate considerable alterations of their N-glycan structures without any negative effect on growth or reproduction. However, glyco-engineering is not a simple process of Knock IN/OUT a gene, and often requires fine-tuning of various parameters to prevent the generation of aberrant glyco-structures inconsistent with the targeted glyco-design. Strategies to overcome challenges included:
- Identification and characterization of possible proteins(s) involved in the process of aberrant glycosylation
- Correct the subcellular localization and expression level of endogenous or recombinant glyco-modifying protein(s)
- Prevent the activity of glycosidases involved in trimming terminal residues from N-glycans
- Increase the accessibility of the glycosite to processing enzymes
Current Projects
Our group’s research focus links Plant glycobiology to Medical Biotechnology.
We study the functional and structural impact of tailored glycosylation on the biological activity of important proteins, e.g. anti-cancer antibodies, cell receptors, antigenic proteins and viral glycoproteins. The outcome of our research can be applied to identify individual structures fundamental for glycan-mediated processes and develop ‘Glycobetter’ proteins with enhanced activities.
We also study the impact of glycosylation on protein-protein interaction axis e.g. during viral infection or in cancer immune evasion. By identifying glyco-variants with improved binding properties we aim to design decoy molecules with potential therapeutic value.
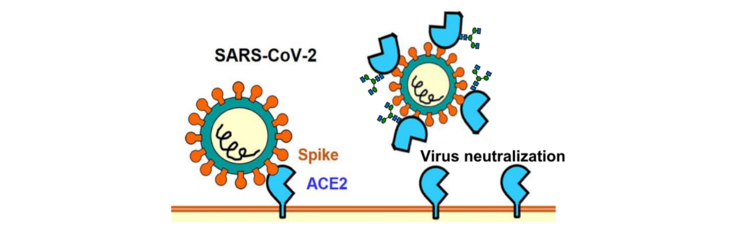
SARSCoV-2 neutralization by plant-derived ACE2
Amongst the various options to block SARSCoV-2 infections by drugs, the inhibition of the binding of the virus to its receptor on human cells (ACE2), is one of the most promising approaches for COVID-19 therapy. Soluble ACE2 reduces virus entry into target host cells by competition with the cellular ACE2 receptor for binding to the SARS-CoV-2 spike protein. In cooperation with scientists of the Austrian "Medicines for Future" our group studies the potential of Plant Molecular Farming for the production of a glyco-optimized ACE2 for therapeutic applications (Medicines for the Future, M4F).
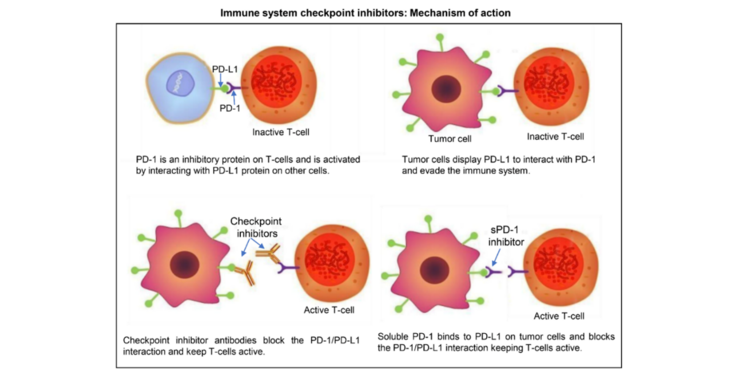
The impact of glycosylation on immune checkpoint inhibitors
Immunological checkpoint inhibitors have emerged as novel therapy option for cancer treatment.
Programmed cell death-1 (PD-1) is protein receptor expressed on the surface of several circulating immune cells (e.g. T/B-cells and natural killer cells), that acts as a “off switch” preventing T-cells from attacking other cells.
It does this when it attaches to its ligands, PD-L1, expressed on a variety of cells, including antigen-presenting cells. Tumor cells can take advantage of these natural protective inhibitory mechanisms by displaying PD-L1 that interacts with PD-1, enabling cancer cells to escape immune surveillance. It has been shown that PD1/PDL1 interaction is mediated by their glycosylation.
The project aims to take advantage of the transient expression system in N. benthamiana and its suitability for glyco-engineering to produce immune checkpoint proteins and antibodies with defined and homogenous human-like glycans. These will be used to determine the functional impact of glycans in the PD-1/PD-L1 interaction. In addition, glyco-engineered plant-derived proteins will be assessed for their binding affinity and effector functions. We expect to be able to identify glycan-optimized proteins that can act as decoy/trap molecules to inhibit the binding of PD-L1 in tumor cells to PD-1 in T-cells to “switch off” the immune system.
Plant-based biotechnological platforms represents a promising innovative and fast way to undertake fundamental studies likely to provide major insights into glycan-dependent interactions helping (i) identifying new biomarkers for early cancer diagnosis and patient stratification (ii) understanding the molecular mechanisms related drug resistance and (iii) guiding novel therapeutic glycan-based strategies to fight cancer.
Previous Projects
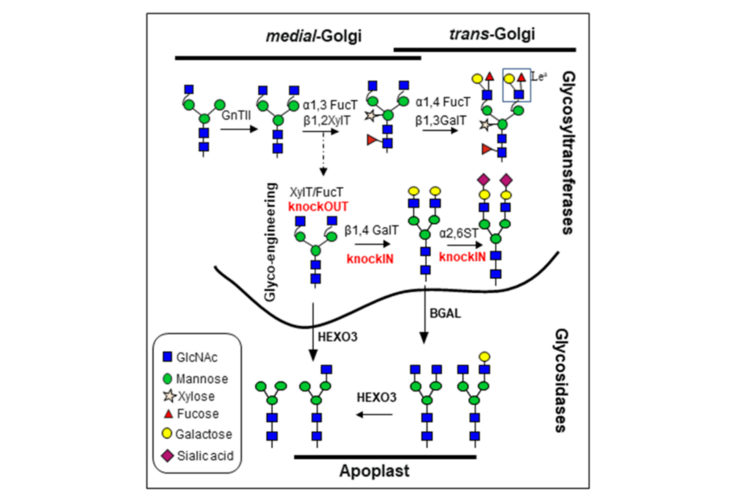
Characterization of N. benthamiana β-galactosidases acting on glycoproteins
Significant progress has been made towards the humanization of protein N-glycosylation in plants either by removing undesirable sugar residues or by introducing the ability to generate non-native N-glycan structures. However, homogenous human-like β1,4-galactosylation is very hard to achieve in recombinant glycoproteins accumulating in the plant-apoplast. Despite the vast efforts to optimize the expression of β1,4-galactosyltransferase (GalT), many glycoproteins still exhibit heterogeneous terminal galactosylation. Here we aim uncover different players that constrict the efficiency of β1,4-galactosylation in plant-derived glycoproteins.
The overall goal of this project is to elucidate the so far uncharacterized mechanisms leading to the trimming of terminal β1,4-galactose residues from N-glycans of secreted proteins. We have identified and characterize N. benthamiana β-galactosidases (BGALs) from the glycosyl-hydrolase (GH) family 35 active in the apoplast. Particularly, studies on their subcellular localization, substrates and specific biological function were not available. We showed that suppression of BGALs expression by RNAi interference or gene editing can be applied to increase human-like galactosylation of several plant-produced glycoproteins.
Collaborations
Dr. Victor Klimyuk:
Icon Genetics, Halle, Germany.
Prof. Renier van der Hoorn:
Department of Plant Sciences, University of Oxford, Oxford, UK
Prof. Celso Reis:
Instituto de Investigação e Inovação em Saúde, Universidade do Porto; Institute of Molecular Pathology and Immunology of the University of Porto (IPATIMUP). Portugal.
Prof. Peter Steinberger:
Division of Immune Receptors and T cell Activation, Institute of Immunology, Center for Pathophysiology, Infectiology and Immunology, Medical University of Vienna, Austria.
Prof. Waranyoo Phoolcharoen:
Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, Thailand.

