Molecular Biotechnology determines and describes the underlying molecular principles of biotechnological production processes. Among these products, recombinant proteins (e.g. biotherapeutics and industrial enzymes) are of paramount importance. To accelerate the development of production strains it is crucial to understand the molecular physiology of the host, and the specific limitations that the product may exert on expression.
The yeast Pichia pastoris (syn. Komagataella spp) has emerged as an efficient host for recombinant protein production. However, folding and secretion of complex proteins is often limited, leading to reduced yields. We focus on the comprehensive understanding of protein folding and secretion, and on cell engineering for the improvement of these processes. Environmental conditions, redox status and core metabolism impact protein production and secretion and therefore represent major research targets of our group.
In the “CD-Laboratory for growth-decoupled protein production in yeast”, the connection between cell growth and protein secretion shall be analyzed by looking at yeast cells cultivated at very low specific growth rates, using the yeast P. pastoris as a model system (link).
Systems biology analyses include genome, transcriptome, proteome, metabolome, and fluxome analyses, these high throughput technologies are performed in cooperation with the Department of Chemistry, FH Campus Wien Bioinformatics group, Metabolic Modelling group, and VBCF. The genome sequences of different P. pastoris strains are available at www.pichiagenome.org. Additionally, also the emerging field of epigenetic regulation is studied.
Subsequently, we apply the knowledgebase generated by these systems biological approaches to design and develop P. pastoris cells capable of synthesizing high amounts of important commercial products, in particular recombinant proteins. Systems biology based cell engineering is combined with detailed understanding of the production platform to further improve protein production in yeasts.
To enable elegant and high-throughput cell engineering strategies, synthetic biology tools such as Golden Gate cloning and CRISPR/Cas9 technology are applied (see also: https://www.addgene.org/kits/gasser-goldenpics ).

Publications of this Research Group
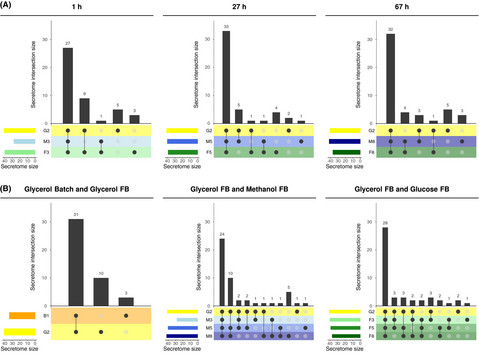
New Publication