Developing a 3D cell culture model to investigate how Gestational Diabetes Mellitus affects fetal Mesenchymal Stem Cells
SUPERVISOR: Cornelia KASPER
PROJECT ASSIGNED TO: Linda JOHNSEN
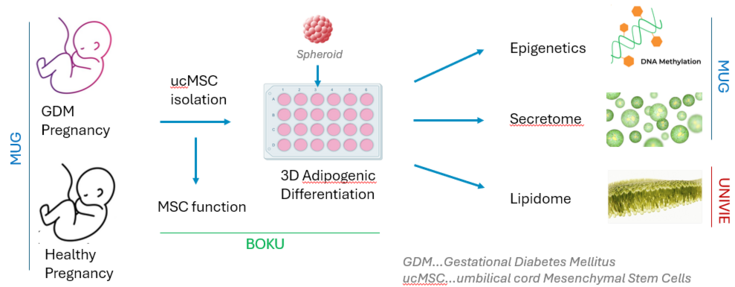
Gestational diabetes mellitus (GDM) stands as the most common pregnancy complication, carrying notable risks for both mother and child. During pregnancy, GDM changes the intrauterine environment, negatively affecting the growth and differentiation of fetal mesenchymal stem cells (MSCs). These cellular changes may predispose the child to metabolic disorders like obesity and diabetes. However, the exact mechanism by which GDM influences MSCs and leads to an increased risk of metabolic diseases in offspring remains unclear. This study aims to explore how GDM might reshape the function of fetal MSCs and MSC-derived adipocytes, potentially hindering the cells' growth, lineage differentiation, and resilience against senescence or cell death after extended proliferation. The MSC-derived adipocytes may display changes regarding ganglioside composition, DNA methylation, paracrine activity, and glucose metabolism. These cellular modifications could play a role in predisposing offspring to metabolic disorders.
To investigate these effects, we will isolate MSCs from the umbilical cords of patients with and without GDM. Initial comparisons of proliferation capacity between these patient-derived MSCs will reveal whether differences arise at the stem-cell level Subsequently, MSCs will be cultured long-term in 3D spheroids to assess their differentiation into adipocytes. We will analyze secreted molecules and their angiogenic potential, ganglioside and lipid profiles, glucose metabolism, DNA methylation, and the transcriptome of these patient-specific MSC-derived adipocyte spheroids. The project is a collaborative effort among the Medical University of Graz (MUG), BOKU University, and the University of Vienna (UniVie), with each team contributing to distinct project tasks.
All cell cultures will be performed under physiological conditions (hypoxia, xeno-free, antibiotics-free) for a better in-vivo translation, contributing to an understanding of how GDM impacts the MSC and adipocyte phenotype and functional properties and how these alterations potentially contribute to metabolic disorders in-vivo. The discovery of these mechanisms may allow for targeted interventions to reduce the long-term health risks associated with GDM, for both the mother and her offspring.