Direct electrochemistry of enzymes via cytochrome domains
SUPERVISOR: Roland LUDWIG
PROJECT ASSIGNED TO: Lan ZHANG
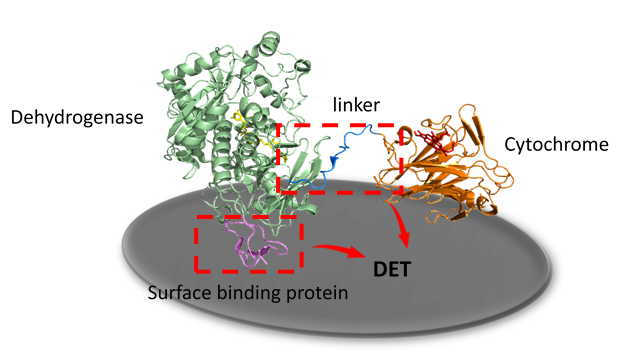
Electrochemical biosensors using enzymes as biorecognition elements are an attractive research topic due to their commercial perspective in healthcare, biotechnology, or the environmental sector. Third generation biosensors, based on the direct electron transfer (DET) between redox enzymes and the electrode are gaining increasing attention due to the simplicity of the sensor architecture and the robust measurement performance. In some DET enzymes, cytochromes function as “built-in” redox mediators to transfer electrons between the enzyme and the electrode surface. The investigated example, cellobiose dehydrogenase, consists of a catalytic dehydrogenase domain and an electron transferring cytochrome domain which are connected by a flexible linker.
To optimize DET rate of cellobiose dehydrogenase, two strategies are pursued. First, the interdomain linker, which governs the distance between the cytochrome domain and the dehydrogenase domain will be investigated, its function characterized and optimized. The aim is to enhance both interdomain electron transfer and DET to the electrode. Second, the optimal orientation and immobilization of cellobiose dehydrogenase on the electrode surface will be studied by attaching surface binding proteins at the C-terminus. Finally, the generated enzymes will be tested in biosensors and electrobioreactors.