Nanoscale triggered release systems for wound dressings
SUPERVISOR: Erik REIMHULT
PROJECT ASSIGNED TO: Giacomo CHIZZOLA
Healthcare-associated bacterial infections of wounds currently represent a high burden to the healthcare sector and patients worldwide. With increasing antibiotic resistance, more effective and refined therapeutic approaches are required. Smart wound dressings, which can respond to stimuli to release an antimicrobial drug, may allow optimised and controlled drug delivery over time without the need to repeatedly open or remove the wound dressing.
A promising tool to allow drug encapsulation and subsequent release within smart wound dressings is represented by vesicles, such as liposomes and polymersomes. Amongst the possible stimuli to be used as triggers, external magnetic fields stand out thanks to their biocompatibility, their capacity for both tissue and wound dressing penetration, and the potential to precisely modulate the excitation.
In this project, hybrid vesicles containing superparamagnetic iron oxide nanoparticles (SPIONs) in their membrane will be developed and optimised to meet the demanding requirements on stability and controlled release posed by their intended application. SPIONs can act as sensitizers for external alternating electromagnetic fields allowing magnetothermal actuation of vesicles purposely engineered to respond to localized thermal stimuli. Optimised vesicles in terms of lipid, amphiphilic block copolymer, and nanoparticle composition will be encapsulated in hydrogels. The project focuses on studying the self-assembly, stability, and magnetothermal actuation of such smart wound dressing hydrogels. They will be tested within the H2020 MSC ITN consortium STIMULUS for their potential to treat burn wound infections without causing antimicrobial resistance or cytotoxicity.
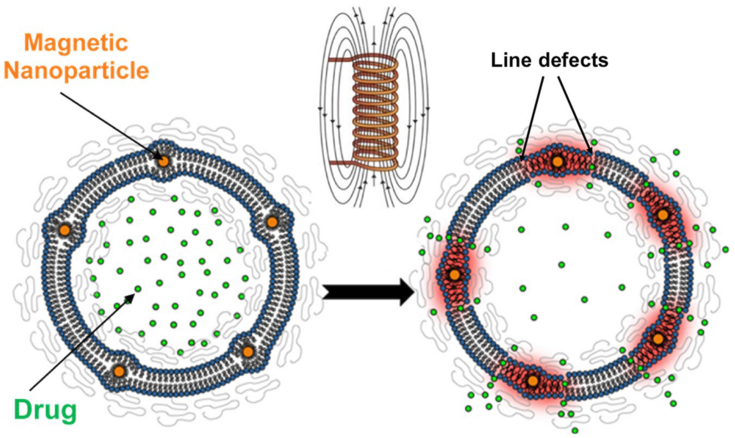
Fig.1 – Schematic representation of cross-section of a hybrid vesicle composed of lipids and SPIONs (magnetoliposome) with polymer brush coating. Upon excitation via external alternating magnetic field (AMF), localised heating induced by the SPIONs via Néel relaxation causes a (local) phase transition of the lipids. The drug encapsulated in the lumen is then able to diffuse outwards at the line defects formed between the two lipid phases. [B. Shirmardi Shaghasemi et al., Sci Rep (2017)]
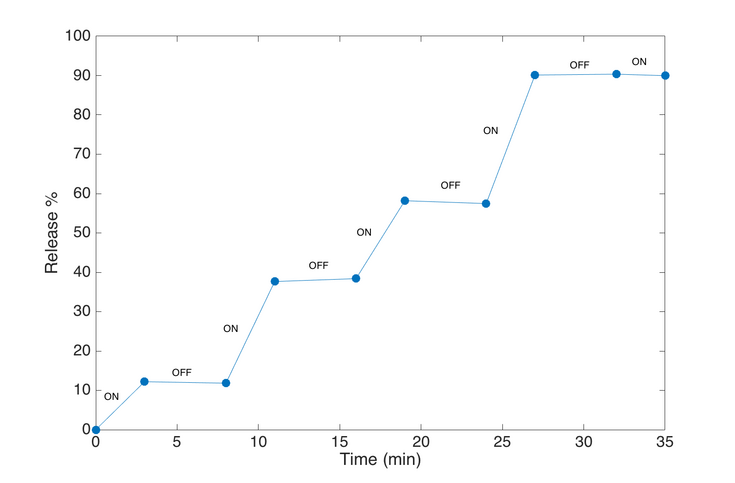
Fig. 2 – Calcein release study with magnetoliposomes (DPPC lipids, 4 wt% 4 nm SPIONs) showing great control on the release via ON (3 min) and OFF (5 min) switching of the AMF. [B. Shirmardi Shaghasemi et al., Sci Rep (2017)]
