SUCCESS STORIES
Lead author publications in reputed Q1 journals
During the summer semester of 2025, Oscar Fabian Garcia celebrated the publication of two articles in highly reputed Q1 journals, both arising from his doctoral research and collaborations within our Doctoral School.
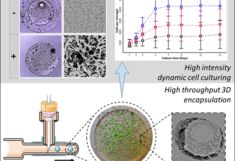
In the first publication, High-Throughput Encapsulation of Stem Cells: Characterizing Dynamic Culture Variability With a Millifluidic Approach (DOI: 10.1002/adhm.202405137 ), Oscar meticulously standardized a hydrogel platform for the dynamic culture of stem cells and characterized their biological variability in 3D environments across multiple material configurations. This versatile, low-cost, and easily transferable technology lays the foundation for future in vitro cell culture studies and exemplifies a successful collaboration between the Institute of Cell and Tissue Culture Technologies (Prof. Cornelia Kasper) and the Institute of Colloid and Biointerface Science (Prof. Eric Reimhult).
The second publication, Effects of Hydrogels on Mesenchymal Stem/Stromal Cells Paracrine Activity and Extracellular Vesicles Production (DOI: 10.1002/jev2.70057 ), provides a comprehensive overview of how hydrogel encapsulation and culture conditions influence the secretion of extracellular vesicles from mesenchymal stem cells. Oscar identifies key challenges in standardization and process control while outlining promising directions for future research in this rapidly evolving field.
These high-impact publications highlight the outstanding results of interdisciplinary collaboration, uniting complementary strengths in cell culture and material science.
Research Stay @ University of Toronto
From September 1, 2024, to February 21, 2025, I, Maximilian Huemer, had the privilege of completing a six-month research stay at the University of Toronto as a Junior Researcher within the framework of my doctoral studies. Under the supervision of Professor Emma Master, I was part of the "Bioproducts Research Lab" within the BioZone facilities. The research group specializes in enzymology, protein engineering, proteomics, and lignocellulose chemistry.
The primary goal of this research stay was to acquire techniques for working with a highly intriguing enzyme known as "lytic polysaccharide monooxygenases" (LPMOs). These LPMOs have the unique ability to modify the surface of cellulose fibers, enabling the development of novel applications.
One such application involves the utilization of a currently underused dye that could facilitate sustainable dyeing processes in the textile industry. Remarkable results were achieved, with dye uptake increasing by over 60% after enzymatic treatment compared to untreated samples.
This exchange not only allowed me to gain numerous new technical skills and specialized knowledge but also helped me develop valuable soft skills. In addition to improving my presentation and discussion abilities, I was able to establish meaningful and lasting connections that will foster future collaborations and exchanges.
I would like to take this opportunity to sincerely thank BOKU DocSchool for enabling me to have this enriching personal and professional experience.

Image 1: Group dinner with the research team
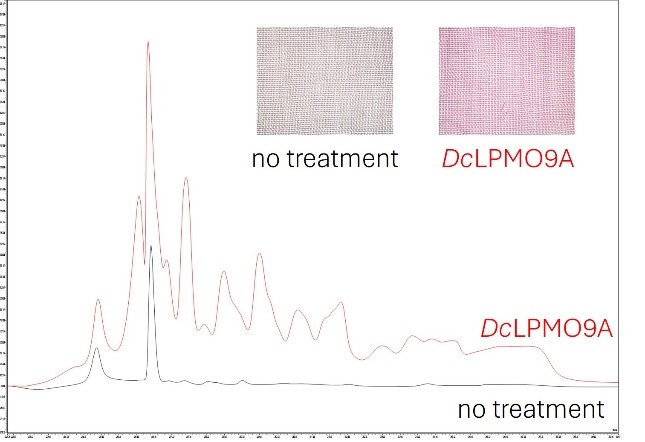
Image 2: Comparison of dye uptake with and without enzymatic treatment
Participation in the HERCULES 2025 Winter School
From March 9 to April 12, 2025, Matthias Weinberger had the privilege of attending the prestigious Higher European Research Course for Users of Large Experimental Systems (HERCULES) Winter School, organized by the Université Grenoble Alpes. This intensive five-week training program is designed for PhD students and early-career researchers in physics, chemistry, and biology, providing advanced knowledge and hands-on experience with large-scale experimental facilities.
The course was primarily held at the European Synchrotron Radiation Facility (ESRF) in Grenoble, France, one of the world’s most advanced synchrotron light sources. As part of the program, Matthias also participated in a research stay at the SOLEIL Synchrotron in Saint-Aubin, near Paris, where he gained hands-on experience with cutting-edge techniques in synchrotron science. The program brought together participants from 25 different nationalities, fostering a truly international and interdisciplinary learning environment. A key feature of the program was a poster session where all participants presented their own research projects and explored the topics of fellow participants. In addition to the academic and technical training, the course also emphasized social activities, such as snowshoeing in the French Alps or group dinners, which helped participants create lasting connections.
Reflecting on the experience, Matthias shared:
"What I liked most about the HERCULES Winter School was the incredible passion for science shared by all the participants. Everyone was so motivated and enthusiastic, which created an inspiring atmosphere throughout the course. I also had the chance to meet many beamline scientists, and these connections have already led to exciting collaborations. The trust and responsibility we were given during the practical sessions were particularly rewarding, as it allowed us to truly engage with the research process.”
This experience represents a significant milestone in Matthias’ academic journey, equipping him with the skills, network, and inspiration to further his contributions to the scientific community.

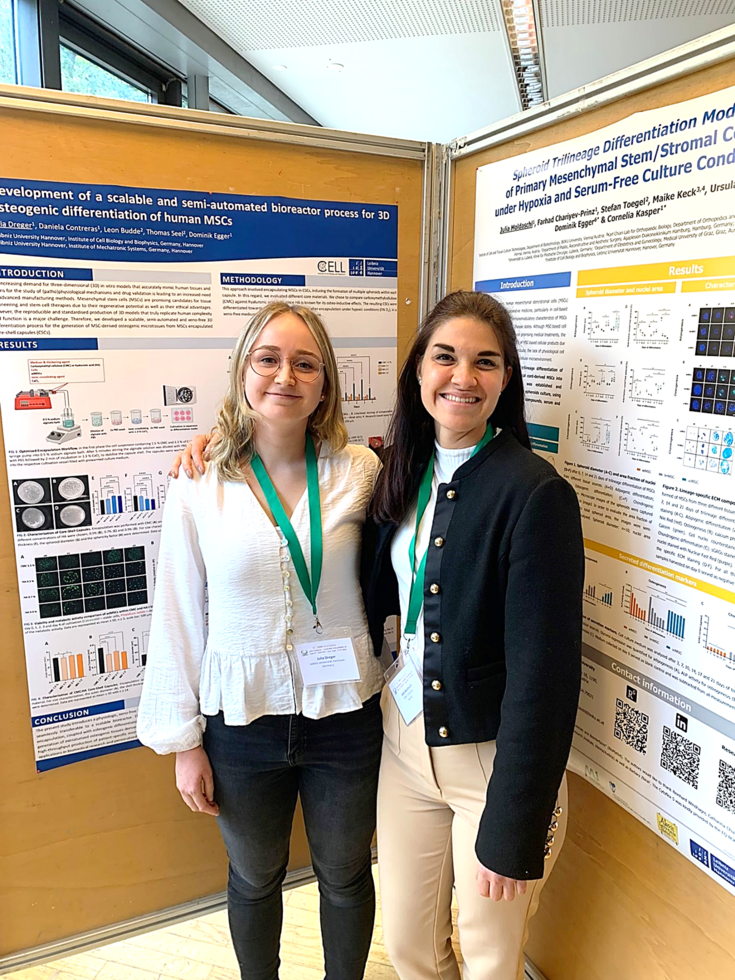
Poster presentation at the ISCT 2025 in New Orleans
In May 2025, Julia Moldaschl participated in the International Society for Cell & Gene Therapy (ISCT) Annual Meeting, which took place in New Orleans. This event marked her first attendance at a major international scientific conference, offering a valuable opportunity to engage with researchers from around the world and gain exposure to the latest developments in the field.
Julia contributed to the conference with a poster presentation named “Establishment and characterization of a physiological human juvenile bone marrow-derived mesenchymal stem/stromal cell line for osteogenic differentiation.” Her work introduces a scalable cell line system that supports 3D spheroid culture under physiologically relevant conditions. This model serves as a promising tool for studying osteogenesis, particularly in pediatric disease contexts, and holds potential for application in drug testing for bone-related disorders. This experience represents an important step in Julia’s academic journey and her growing involvement in the international research community.

Short talks at ÖGMBT annual events
In July 2025 and September 2024, Oscar García presented his work at the Workshop „The Evolving Landscape of Cell Culture: Advancing Technologies and Future Prospects” and the Annual ÖGMBT Meeting, where he discussed the “Encapsulation of Mesenchymal Stem Cells for Dynamic Culture using a Scalable Milli-Fluidic System” and the “Impact of crosslinking conditions on microgel-encapsulated mesenchymal stem cells in small scale bioreactors”. These events offered opportunities for networking with scientists and industry professionals, focused on advances in stem cell research, tissue engineering, and cell-based product development. The ÖGMBT annual meeting also summons top experts for exchange, showcasing the work of early career researchers, helps the attendants to get a notion of the field beyond the horizon of their own specialization, and shows what is happening in Austria in the field of life sciences.
Oscar’s work establishes a robust, scalable, and biologically relevant system for culturing MSCs, addressing a major bottleneck in their clinical translation and biomanufacturing. The synergic combination of bioreactor technologies and milli-fluidic encapsulation of stem cells improves both the scientific understanding and practical feasibility of stem cell-based regenerative medicine. It brings MSC’s culturing protocols to a physiologically relevant state that better incorporates the natural cues of their native niche, remaining viable and functional under dynamic conditions, and showing that the system is ready for integration into scalable bioprocesses, bridging the gap between laboratory research and real-world production.
First Lead Author Publication on MSC Spheroid Differentiation
Julia Moldaschl celebrated a significant achievement in July 2024 with the publication of her first lead authorship article in the journal Frontiers in Bioengineering and Biotechnology. The research article, titled “Spheroid trilineage differentiation model of primary mesenchymal stem/stromal cells under hypoxia and serum-free culture conditions”, represents an important step in her scientific development.
In this study, Julia presents a scaffold-free 3D differentiation model for primary mesenchymal stem/stromal cells (MSCs) derived from bone marrow, adipose tissue, and umbilical cord. Cultivated under hypoxic and serum-free conditions, the platform supports consistent spheroid growth and effective trilineage differentiation. The model offers a physiologically relevant system for studying MSC behavior and holds potential for future applications in tissue engineering and disease modeling, particularly in the context of bone sarcomas.

During the ÖH election in May 2025, BioMatInt’s doctoral candidate Konstantin Nikolaus Beitl was elected as one of the Doctoral & PhD students’ representatives (DokStV, ÖH BOKU).
Together with three colleagues, Victor Gonzales-Mallen, Larissa Hofer, and Thomas Keller, they represent all doctoral and PhD students at BOKU for the next two years.
The DokStV advises and assists students of all doctoral and PhD programs, and provides guidance and support. If you need any help navigating through academic and administrative challenges regarding your doctorate or PhD, don’t hesitate to contact your representatives!
stvdoktorat(at)oehboku.at

In March 2025, Konstantin Nikolaus Beitl successfully published an article titled “Synthesis, Microbiology, and Biophysical Characterization of Mutanofactins from the Human Oral Microbiome” in ACS Central Science. The paper marks a significant scientific breakthrough enabled by close collaboration between BOKU University and ETH Zürich. Funded by WWTF, the study will be a core part of his dissertation and features equal contributions from Konstantin Nikolaus Beitl and Leon Thies (BOKU), and Lukas Lüthy (ETH Zürich). Corresponding authors are Christina Schäffer (BOKU), Erick Carreira (ETH Zürich), and BioMatInt faculty member Erik Reimhult (BOKU).
Publishing in a high-impact journal highlights the excellence of collaborative research, merging strengths in synthetic chemistry, microbiology, and biophysics.
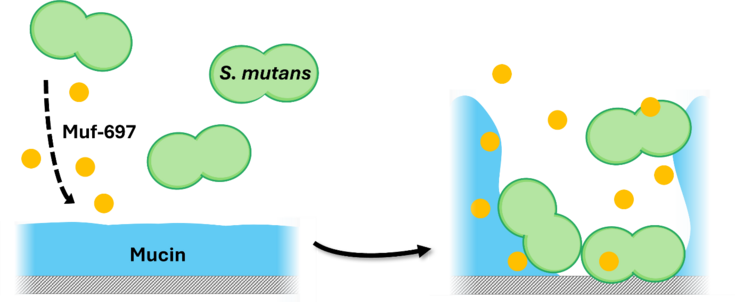
Key scientific advances include the first-ever total synthesis of the complete mutanofactin family, enabling investigations into their role in promoting the formation of dysbiotic bacterial biofilms. The study provides unprecedented biophysical insights, showing how mutanofactin 697 alters mucin layers in the human oral cavity to aid bacterial adhesion to this protective barriers. These findings advance our understanding of dental caries pathogenesis by revealing how mutanofactins support bacterial colonization on teeth. The research not only enhances basic scientific knowledge but also lays the groundwork for novel anti-caries strategies, potentially targeting mutanofactin-mucin interactions.
https://pubs.acs.org/doi/10.1021/acscentsci.4c02184
Biofilms 11 Conference, 13–15 May 2025 in Cardiff, UK
BioMatInt doctoral candidate Konstantin Nikolaus Beitl and faculty member Dr. Guruprakash Subbiahdoss have recently presented their latest research at the Biofilms 11 Conference, held from 13–15 May 2025 in Cardiff, United Kingdom.
Mr. Beitl participated in the poster session, with a poster titled “Mutanofactin affects interactions of mucin-coated surfaces and Streptococcus mutans”, presenting exciting results from his work on how Streptococcus mutans (S. mutans) adheres to saliva-coated surfaces in the human oral cavity with the help of a newly discovered lipopeptide called mutanofactin. This small-molecule secondary metabolite produced by S. mutans allows this pathogen to build dysbiotic biofilms on teeth. Such biofilms can transform into dental plaque and cause tooth decay, one of the globally most prevalent chronic deseases. Key finding in his study was that one type of mutanofactin, Muf-697, irreversibly interacts with mucin, a glycoprotein and major component of saliva, which forms a natural barrier against bacterial adhesion. This interaction between Muf-697 and mucin induces major structural changes in the mucin layer, allowing S. mutans to overcome this protective barrier and attach itself to our teeth.
This ongoing project is a successful collaboration between BOKU University and ETH Zürich funded by the WWTF, and has already yielded a high impact publication in ACS Central Science (https://pubs.acs.org/doi/10.1021/acscentsci.4c02184).
Dr. Subbiahdoss also contributed a poster, as well as a flash talk titled “Battle for the Surface: Staphylococcus Biofilms vs Osteoblast Adhesion on DNA Polyelectrolyte Multilayer Coatings”. These contributions highlighted a critical investigation into the competitive dynamics between host cells and bacterial pathogens on biomaterial surfaces.
This study explores how DNA-based polyelectrolyte multilayer coatings influence the adhesion of osteoblasts and their ability to outcompete Staphylococcus biofilms, notorious for causing implant-associated infections. This research provides important insights into the design of multifunctional coatings that can promote tissue integration while resisting bacterial colonization — a key challenge in the development of next-generation medical implants.
This work has also been published in a special issue of the journal Colloids and Surfaces B: Biointerfaces (https://doi.org/10.1016/j.colsurfb.2024.114336) dedicated to the retirement of Professor Henk J. Busscher, under whom Dr. Subbiahdoss completed his Ph.D. and postdoctoral research at the University of Groningen, Netherlands. This study was made possible through funding from the Hochschuljubiläumsfonds der Stadt Wien, with support from BOKU University, Vienna.


Publication of Second Research Article
In October 2024, Ilias Nikolits celebrated the success of publishing his second research article, titled "Characterization of MSC Growth, Differentiation, and EV Production in CNF Hydrogels under Static and Dynamic Cultures in Hypoxic and Normoxic Conditions", in the journal MDPI Bioengineering. This publication marks another milestone in Ilias' PhD journey, bringing him closer to completing his doctoral studies at BOKU.
The peer-review process was highly constructive, with positive feedback that contributed to the refinement of an already solid piece of research. Ilias expresses excitement and pride in seeing his work recognized in the scientific community, which further supports his ongoing efforts in understanding the behavior of mesenchymal stem cells (MSCs) within cellulose-based hydrogels. The paper represents a significant contribution to the field of cell-based therapies and biomaterials, showcasing innovative approaches in advanced cell culture techniques.
With the successful publication of this article, Ilias is not only closer to achieving his PhD but also excited for the opportunities this research will open up in the future.


Studying Spider Silk for Nerve Repair: Overcoming Challenges in Synchrotron Experiments

In the quest to improve nerve regeneration for patients suffering from injuries to the peripheral nervous system, spider silk has emerged as a promising material. This remarkable natural fiber can be used as a filler in nerve guidance conduits, helping nerves regenerate more effectively. Imagine a silk-filled implant placed in the body between two damaged nerve ends, supporting the body’s natural healing process.
The goal of my research is to understand the structure-function relationships of natural spider silk, which is key to producing it artificially for medical use. To do this, I’m investigating how mechanical stress and strain impact the silk’s inner crystal structure.
One of the most exciting experiments we conducted involved testing the silk fibers under mechanical strain using X-rays. This was done at two world-leading synchrotron facilities—MAX IV in Sweden and the ESRF in France. By stretching single spider silk fibers while exposing them to a focused X-ray beam, we were able to observe how the structure inside the silk changes under tension.
This was no easy task! The X-ray beam, with a focus as small as 100 nanometers, is so powerful that it can damage the delicate silk fibers. We had to carefully adjust our scanning parameters to avoid cutting the fibers with the beam. Nevertheless, we successfully tested more than 100 individual silk fibers (including several night shifts), both in ambient conditions and at varying levels of humidity.
The results of this study bring us one step closer to understanding the unique properties of spider silk. By unlocking these secrets, we hope to design artificial silk that can improve the success of nerve regeneration in the future—giving patients better outcomes and reducing suffering.
5th European Summer School on Microfluidics in Catanzaro, Italy
In September 2024, Elettra Verin and Oscar García attended to the 5th European summer school on microfluidics at the University “Magna Graecia” in Catanzaro, Italy. The training strengthened their knowledge on biomaterials for cell culture, and expanded their horizons on microfabrication, in-line analytics, organs on a chip, visualization and cell therapy in microfluidic devices, among other selected topics from experts on the field. The summer school also offered hands-on sessions and several networking opportunities with peers and industry partners.
Elettra, who recently joined our doctoral school, presented her project “Optimization of a 3D analysis platform for the assessment of cartilage graft maturation in vitro”, which provides an alternative to animal testing for cartilage regeneration therapies. Her project uses concepts from miniaturization and in-line sensors, developing a bioreactor platform to timely provide information on the maturity of developing cartilage grafts, sampling different metabolites from within and outside the cultured tissue. Oscar, who is on his third year at our doctoral school, presented the results of his project “Establishing a high throughput fluidic system for mesenchymal stem cells encapsulation and expansion”, which enables the generation of plentiful low cost and high fidelity microgels, to observe proliferation and metabolic activity of adherent cells in dynamic culturing systems. His technology has been successfully applied to stem cell differentiation and manufacture of extracellular vesicles.
“We are grateful that the doctoral school gave us such an opportunity, which for sure contributes to the accomplishment of our scientific goals and connects us with our research community, we also encourage our colleagues to join to the next instalment of the microfluidics summer school which will be held in Denmark in 2025”.

Flash talk and Poster - ÖGMBT Meeting 2024
Julia Moldaschl was honored to be accepted for a flash talk and poster presentation at the 16th ÖGMBT Annual Meeting in Graz, held on 17th September 2024. She was thrilled by the opportunity to participate in such a renowned event and looked forward to sharing her latest research and connecting with fellow scientists. However, due to unforeseen weather conditions and train delays caused by heavy rain, Julia was unable to arrive in time for the flash talk. Despite this unexpected challenge, the day turned out to be incredibly rewarding.
Julia fully embraced the opportunity to present her research during the poster session, where she had lively and enriching discussions with other scientists. Her poster, titled „Spheroid Trilineage Differentiation Model of Primary Mesenchymal Stem/Stromal Cells under Hypoxia and Serum-Free Culture Conditions” sparked meaningful exchanges, and she connected with new colleagues in the field – as visible on the picture.
Although the journey did not go as planned, the day still proved to be a fantastic success, filled with collaboration, inspiration, and fresh perspectives for the future.
RÅC International Summer School 2023
Here are some highlights of my experience at the RÅC International Summer School 2023, which took place from 20 to 27 August 2023 in Lüneburg, Germany. The central theme of this event was "Cutting-edge Neutron and X-ray Research for a Sustainable Future". During my time at the Summer School, I had the privilege of presenting a poster entitled "Nanobeam X-ray Diffraction on Various Spider Silk Fibres". I am particularly grateful for the opportunity to interact with fellow participants from different parts of the world. To further enrich my participation, I took part in a Science Slam presentation where I shared my insights on spider silk. The overwhelmingly positive response from the audience was truly inspiring. In addition to my own contributions, the Summer School provided an invaluable opportunity to attend a series of cutting-edge scientific lectures by renowned scientists in their respective fields from Germany and Sweden. The Summer School broadened my intellectual horizons, rekindled my passion for materials science and strengthened my commitment to sustainable solutions.

Poster Prize - Sabrina Nebel
“Life Sciences and cutting-edge technologies” was this year’s topic of the annual meeting (2023) of the Austrian Association of Molecular Life Sciences and Biotechnology (OEGMBT), which took place from 19th-21st of September in Salzburg, Austria. A broad palette of topics was covered, which included current developments in applied and medical life sciences. In this context, also basic research was represented by a diverse set of talks. The organizers struck a good balance between internationally renowned speakers and young researchers, allowing them to showcase their work in front of a large crowd. Of the 62 high-quality oral lectures, 2 were given by DocSchool students (Julia Moldaschl, Ilias Nikolits) and 3 of the 71 posters came from students of the BioMatInt program. In the final award ceremony, the poster presentation by Sabrina Nebel was awarded one of the three prizes for an outstanding poster presentation kindly sponsored by The FEBS Journal. We congratulate!
Further information
https://febs.onlinelibrary.wiley.com/hub/journal/17424658/features/the-febs-journal-poster-prize-

Editor’s Choice for Core-Shell Capsules
The Article "Alginate Core-Shell Capsules for 3D Cultivation of Adipose-Derived Mesenchymal Stem Cells”, published in February 2022, was recently selected as "Editor's Choice". The paper was part of the Special Issue “Material and Engineering-Based Approaches for Organoids” in the Open Access journal MDPI Bioengineering and was the first lead-authorship publication of Sabrina Nebel, doctoral candidate at the Institute of Cell and Tissue Culture Technologies.
Sabrina’s research is focused on mesenchymal stem cells (MSCs), progenitor cells of the connective tissue that can be found in almost all tissues, even in adults. These cells are easily available and can be used in a wide variety of cell therapies and in-vitro testing systems. However, since millions of cells are required for any application, ex-vivo cell expansion is inevitable. Cultivation in petri dishes in 2D is still considered the gold standard, despite evidence that 3D cultivation not only retains but can even improve the beneficial properties of the cells.

Part of Sabrina’s PhD project is to develop such 3D cultivation systems. In her publication, she presents an approach in which the cells are enclosed in so-called core-shell capsules. These capsules consist of an outer barrier made from alginate and an inner, liquid compartment. This core contains the stem cells which can then form direct cell-to-cell contacts just as in their physiological environment. The alginate shell serves as a protective layer from mechanical stress and allows for nutrient and waste diffusion.
“I like to joke that I am making ‘Stem Cell Bubble Tea’ in the lab, as my capsules are made the same way as the popping bubbles you can find in lots of these tea shops” she explains. They are of course not edible, but will hopefully be a valuable tool for stem cell expansion in the future, paving the way for novel therapies.
Conference Talk at the 15th ÖGMBT Annual Meeting
Ilias Nikolits gave his first conference talk at the 15th ÖGMBT Annual Meeting in September 2023, held in Salzburg, Austria. As part of the "Regenerative Medicine & Stem Cells" session, Ilias presented his research titled, "Effects of different in vitro cultivation conditions on human mesenchymal stem cells encapsulated in TEMPO-oxidized nanofibrillated cellulose hydrogels." His talk focused on recent findings from his PhD work, which explores how varying cultivation environments impact MSC growth, differentiation, and extracellular vesicle production in cellulose-based hydrogels. This study is particularly relevant for improving cell-based therapies and in vitro testing approaches.
“This was my first experience presenting at an international conference, and it was both exciting and rewarding. I felt a great sense of pride in being able to share my findings with leading experts in the field. Engaging with fellow researchers and receiving feedback was incredibly valuable, and it has inspired me to continue pushing the boundaries of my research. Overall, the experience strengthened my confidence in public speaking and allowed me to showcase the progress and potential impact of my work.”

Talk at ÖGMBT Annual Meeting
Julia Moldaschl is a doctoral candidate at the Institute of Cell and Tissue Culture Technologies (ICTCT) at the Department of Biotechnology since September 2021. One of her most exciting achievements so far was her contribution to the 14th ÖGMBT Annual Meeting, in Vienna in September 2022. She gave a talk titled “Multilineage Differentiation of Juvenile Primary Bone Marrow-Derived Mesenchymal Stem Cells in 3D under Normoxic and Physioxic Conditions” in the section “Cell Based Assays, Therapies and Products”. This posed an ideal opportunity for Julia to share her experience with mesenchymal stem/stromal cells (MSCs) cultured and differentiated under physiological conditions.
In particular, her research focuses on the establishment of a MSC multilineage differentiation model under physiological conditions, which comprise scaffold-free 3D culture approaches, reduced oxygen conditions, that can also be considered as “Physioxia”, and the application of xeno- and serum-free media.

Instead of broadly used 2D cultivation systems, Julia cultures MSCs as three-dimensional aggregates (spheroids) in micropatterned multi-well plates. That culture system enables the formation of uniform spheroids, allows for up-scaling and has been shown to lead to increased stemness and enhanced differentiation capacity for certain lineages. Oxygen conditions of MSCs´ natural microenvironment are aimed to be resembled by providing physioxic culture conditions. As the third major parameter, Julia is only using xeno-free and serum-free media instead of Fetal Bovine Serum (FBS) supplemented media. This selection is associated with increased proliferation rates and the elimination of ethical problems and risks of pathogen transmission originating from FBS. The application of all three mentioned culture conditions aims to recapitulate the in vivo situation of the cells and therefore increases the relevance of data derived from in vitro models, particularly in the context of MSC-based therapies.
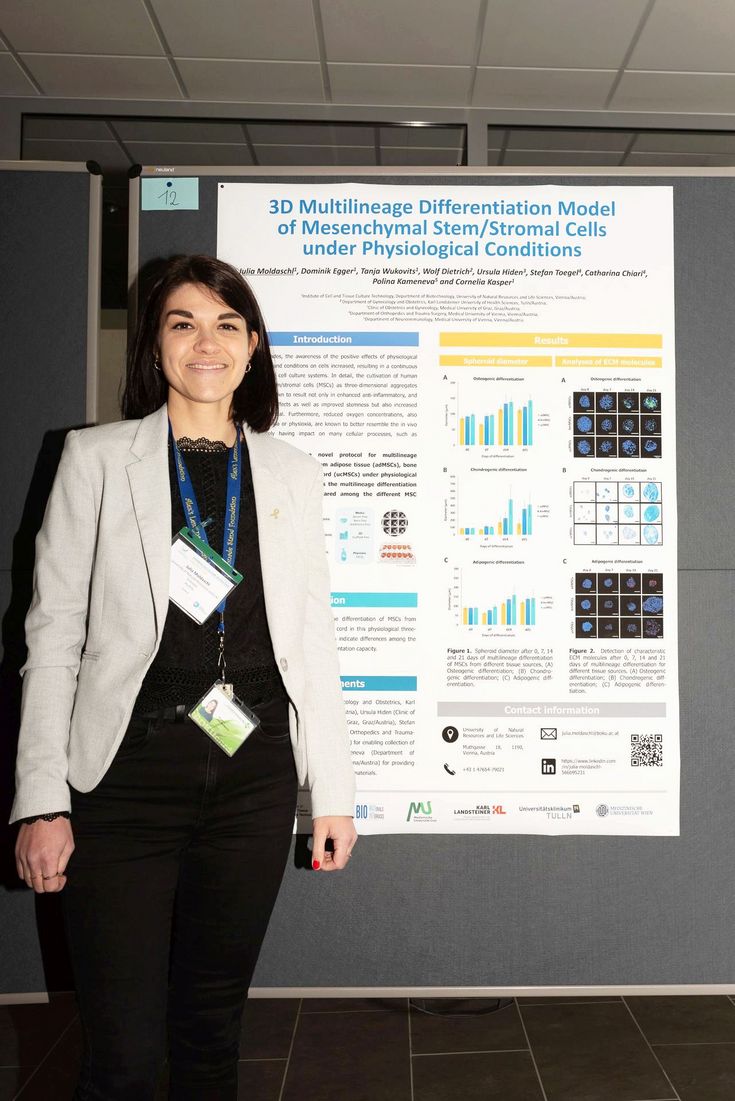
Poster Prize – ÖGMBT Workshop 2023
On 17th March 2023 Julia attended the ÖGMBT Workshop “Advanced Cell Culture Technologies” and was awarded the prize for the best poster. The workshop was organized by the ÖGMBT working group “Cell based assays, therapies and products” and took place directly at BOKU. During the workshop Julia joined lively discussions, exchanged experience in 3D cell culture technologies and connected with new colleagues. “It is such an enriching experience! I got helpful feedback on my work and so many questions that benefited my thesis writing”. On top of this exciting day, Julia won the poster prize for her poster entitled “3D Multilineage Differentiation Model of Mesenchymal Stem/Stromal Cells under Physiological Conditions”.
First time presenting at a conference
Karolina Peter, member of the Doctoral School Biomaterials and Biointerfaces, could engage many interested listeners with her scientific presentation at the JUNIOR EUROMAT conference (19-22 July, Coimbra, Portugal). The presentation, titled "Zooming inside spider silk" - Using synchrotron nanoXRD to compare the ultrastructure of spider silks." was very well received and motivated many of the audience to ask interested questions. There were research results presented, which were collected at the Institute of Physics and Materials Science (BOKU).
Moreover, this was the first conference for Karolina that was not held in a virtual setting, and her first oral presentation.In addition to numerous other interesting lectures, one could also participate in workshops such as Electron Microscopy or Horizon Europe. In particular, the contact with other young scientists was great at JUNIOR EUROMAT 2022 in Coimbra. All in all, a very successful experience, both personally and scientifically valuable.

Research at a large-scale facility

Everything started with a remote experiment at the end of 2020, to which the team from the Institute of Physics and Material Science (IPM) was unfortunately unable to go in person due to Covid-19. Then, in June 2022, the time had finally come, and it was possible to travel in person to the ESRF (European Synchrotron and Radiation Facility) in Grenoble, France. The proposal for an experiment on spider silk was accepted, which is reason to celebrate.

After extensive preparation of samples (single fibers from different spiders as well as microtome sections of the same), the goal was to learn as much as possible about the ultrastructure of spider silk using nanobeam X-ray scattering. Also, thin sections of spider silk, which allow to look at the differences in the structure in a locally resolved way. On site, the team from IPM was supported by the scientists at the beamline (ID13 nanofocus beamline) and accompanied during the experiment. In total, the experiment lasted for 5 days, and a very large amount of data was collected, all of which is now waiting to be evaluated and analyzed. The team consisted of Karolina Peter (main responsible for the experiment), her colleagues Leon Ploszczanski and Arno Frank. Even though after the experiment the need to "catch up" on sleep and recharge the batteries was large (the synchrotron is in operation 24/7), it was an unforgettable and exciting experience, which will soon also result in an interesting publication.
My year of 2022 together with BioMatInt - Johannes Stöckelmaier
When I joined BioMatInt in 2021, I didn’t expect the changes it would bring to the path of my PhD-project. While I needed the first months to get familiar with topic and literature, BioMatInt gave me the opportunity to visit and learn from colleagues and research institutes abroad, leading to valuable gains in knowledge.
While I boarded my flight from Vienna to Copenhagen on March 1st, I knew that I would get to meet one of the most renowned professors for computer simulation of intrinsically disordered proteins in Europe. However, I did not expect to also find a group of great colleagues and a progressive and beautiful city to live in. There is a lot to learn from the city of Copenhagen, especially how to build a top-notch biking infrastructure; the best I have ever seen in my life.
I was able to significantly progress my PhD project, specifically in regard to ensemble reweighting; a topic the Lindorff-Larsen research group has vast experience in. I was given time to think through the mathematical details of their method and to experiment with their data.
During my stay in Copenhagen, I learned:
* … how to use the BME-Reweighting tool.
* … the workflow of a RNA reweighting-example.
* … insight into methodology and mathematical background of the BME method.
* … insight into danish culture and a (little) bit of their language


After visiting Scandinavia in the northern part of Europe, I had the chance to join a one-week long seminar about disordered proteins at Institut d'Etudes Scientifiques de Cargèse. Located on the island of Corsica, France, it was an adventure to get there with in-between stops in Paris, Nice and Ajaccio. The seminar was hold at a very remote location directly on the shore of the Mediterranean Sea. I was able to meet more than 30 like-minded colleagues from Europe, the US and from Japan. Lectures and trainings where held by senior scientists from France, Germany and the United States.
While joining the seminar, I learned:
* … how to use an ensemble refinement method.
* … how to use Flexible-Meccano to generate conformational ensembles.
* … how to approach IDP phase-separation simulation.
I’m very glad that BioMatInt gave me opportunity to take part in those events and I look forward to an equally successful 2023.
ATTENDANCE GRANT – ESCMID/ASM CONFERENCE 2022
In October 2022, Goodness Osondu-Chuka won an attendance grant to participate in and present her Ph.D. work at the ESCMID/ASM Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance (2022), which took place in Dublin, Ireland.
Goodness is a doctoral candidate of the BioMatInt at the Institute of Biologically Inspired Materials. In her project, she creates a three-dimensional biofilm model that replicates key aspects of the biofilms found in the lungs of individuals with cystic fibrosis, a chronic illness without a cure. Through this model, she intends to investigate and comprehend the protective functions of biofilms in persistent infections, particularly in the lungs of cystic fibrosis patients, that prevent effective antibiotic treatments.
She applied for this grant because it offered an excellent opportunity to share and gain more knowledge on various aspects of microbial biofilms as well as novel techniques and approaches for solving the problem of antimicrobial resistance and tolerance in chronic infections. The grant allowed Goodness to present her first results on extracting, reconstituting, and printing the extracellular polymers that provide the structural properties of cystic fibrosis biofilms.
The conference, which was focused on the challenges of developing new agents and solutions for antimicrobial resistance, started with a half-day boot camp designed to share the wealth of experience from the community. This was followed by two and a half days of high-quality symposia, roundtable discussions, keynote, and young investigator lectures. The meeting was designed to bring together researchers, academics, industry regulators, and funders to share experiences, collaborate and learn. In her own words, “Attending the conference was a fantastic chance to stay informed about current research, express my thoughts, and network effectively. I eagerly anticipate similar opportunities to attend and actively participate in future conferences”.

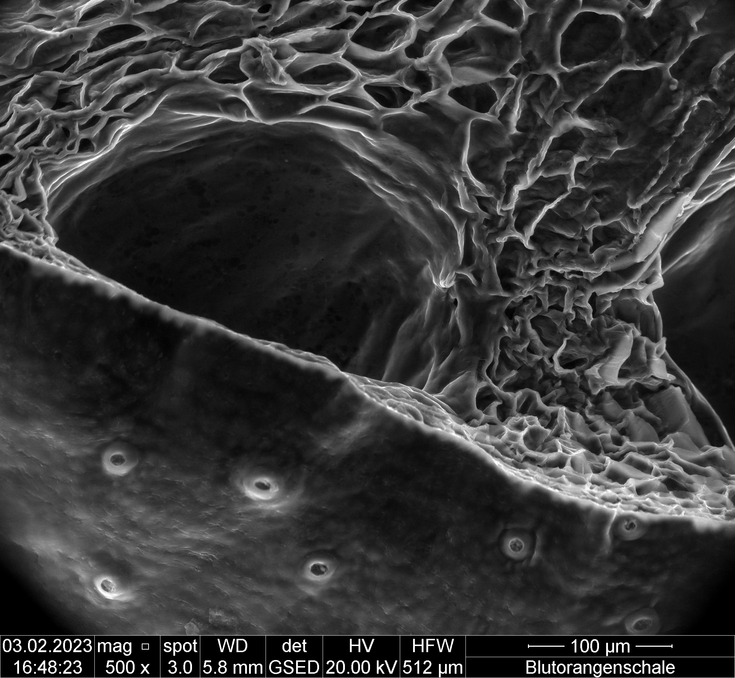
Testing single spidersilk fibers at various humidity conditions - Leon Ploszczanski
The ESEM has not been used for the last two years. I had to completely skip and clean the water cooling to get the brown algae out of the pipes. But the result is very satisfying, you can examine a fresh organic sample without much drying effects in environmental mode with water vapour at 400 Pascal pressure. Between the sample, cooled to 4 °C, and the electron beam outlet are water molecules that protect the sample from drying out and also guide the secondary electrons to the detector. In the pictures you can see the setup in the chamber of the ESEM and in the micrograph a vacuole filled with essential oil in the outer shell of a blood orange.
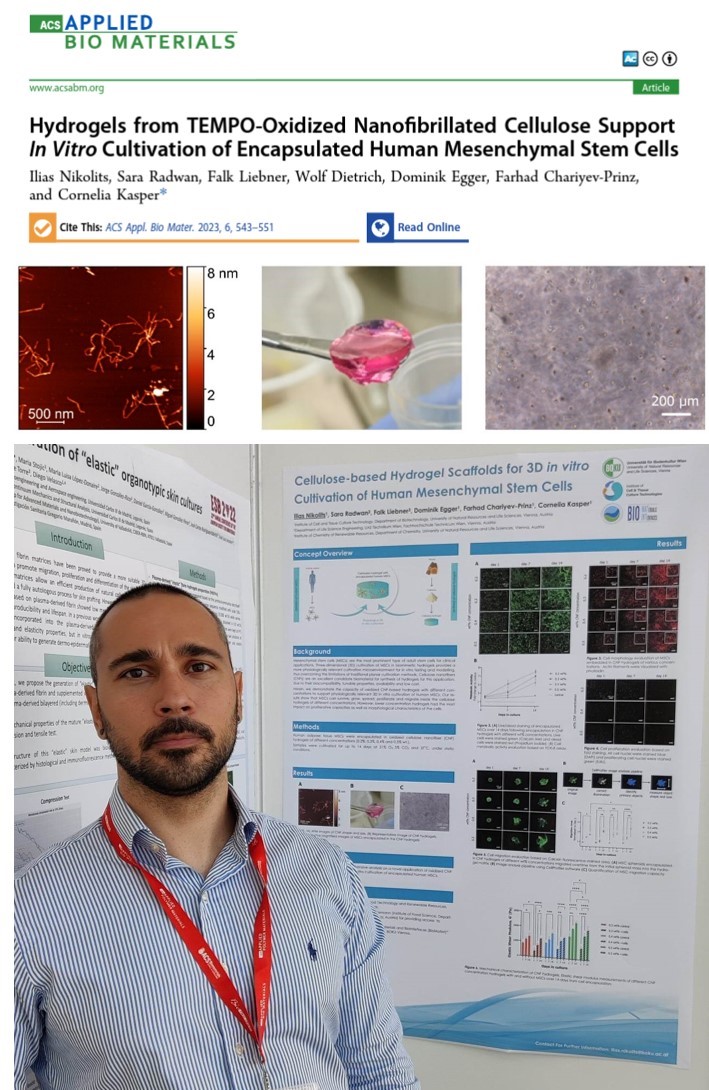
First lead authorship publication
On February 2023 the article “Hydrogels from TEMPO-Oxidized Nanofibrillated Cellulose Support In Vitro Cultivation of Encapsulated Human Mesenchymal Stem Cells” was published on the ACS Applied Bio Materials journal. This was the first lead authorship publication of Ilias Nikolits, doctoral candidate at the Institute of Cell and Tissue Culture Technologies (ICTCT) at the Department of Biotechnology of BOKU.
The aim of Ilias’ PhD project is on the development of hydrogels for 3D in vitro cultivation of human mesenchymal stem cells (MSCs). The material he is focusing on his research is cellulose, a highly abundant and inexpensive natural polymer, with high biocompatibility and tunable properties. The hydrogel scaffolds synthesized from cellulose are aiming to provide a 3D in vivo-like environment during cultivation of MSCs in vitro, compared to the gold standard 2D plastic adherent methods, by preserving the physiological characteristics and functions of the cells.
In this publication, Ilias shows that hydrogels synthesized from chemically functionalized cellulose nanofibers consist a very promising platform for in vitro cultivation of humans MSCs. Encapsulation of MSCs in those hydrogels mimics a 3D tissue-like environment and cells can perform their physiological functions and behavior.
“When people ask me what hydrogels are, I give them the example of Gummy Bears. So, I am basically putting stem cells in Gummy Bears made from wood powder. But is seems the cells really like it” -Ilias quotes.

Research stay abroad - Magnetically triggered drug nanocapsules in hydrogels
University of Siegen, Germany (February 2023)
In February 2023, Giacomo Chizzola spent a month at the University of Siegen, Germany, to expand his knowledge and abilities in polymer synthesis. Giacomo, a member of the Biomaterials and Biointerfaces Doctoral School, is a doctoral candidate at the Institute of Biologically Inspired Materials.
His research focuses on developing the building blocks – specialised nanoparticles, oligomers, and polymers – as well as the strategies to achieve release of encapsulated drug compounds via magnetothermal actuation. To this end, Giacomo visited the partner laboratories of Professor Holger Schönherr and Professor Ulrich Jonas at the University of Siegen, in order to learn the synthesis procedures of some state-of-the-art polymers.
One of the polymers he produced is poly(ethylene glycol)-block-poly(lactic acid) (PEG-b-PLA), which is obtained via ring opening polymerization of cis-lactide onto a PEG macroinitiator. The synthesis can be adjusted to yield PLA blocks of varying lengths, which in turn result in different assembly architectures of the block copolymer in water. A second polymer that Giacomo worked on is poly(acrylamide-co-acrylonitrile), which was chosen for its tunable thermoresponsive properties. To have the best control over the polymer growth and critical solution temperature, he learned how to perform a sophisticated polymerization reaction called reversible addition-fragmentation chain-transfer (RAFT) polymerization.
Giacomo’s guest research stay in Siegen was enrichening and successful, as he came back to Vienna with a few different polymers to further modify and test for their properties and performance. He expects to return to Siegen later in the year or next year to learn more and produce more polymers tailored specifically to his research goals. Once back in Vienna, Giacomo commented the following:
“What I truly enjoy about my research project and being part of the BioMatInt Doctoral School is being immersed in such an interdisciplinary environment. My stay in Siegen was, in many ways, a true masterclass in polymer synthesis, a field in which I had very limited practical experience beforehand”.