Engineering of Oxidoreductases for Lignocellulose Processing
SUPERVISOR: Georg M. GÜBITZ
PROJECT ASSIGNED TO: Verena BRAUNSCHMID
Laccases (EC 1.10.3.2) are some of the earliest studied enzymes, while many innovative applications have been reported recently1. In nature, laccases oxidize a multitude of different substrates and are involved in many processes such as plant defense mechanisms, fungal pathogenesis, lignin degradation and polymerization2. Lignin is the second most abundant biopolymer on Earth, surpassed only by cellulose. It is a by-product of the pulp and paper industry. Only 2% of the annually produced 50 million tons of lignin are used for value-added applications3. Although laccases are natively able to polymerize lignin, when supplied with oxygen4, they are not “designed” to meet industrial process requirements. Therefore, heterologous expression and genetic engineering of laccases have become valuable tools to make them efficient biocatalysts for industrial applications. Genetic engineering might include the addition of binding domains or mutations of crucial sites to enhance substrate binding and catalytic activity. The addition of a substrate-binding domain to a novel laccase was already shown to increase its lignin polymerization ability. This study aims at finding, designing and applying novel laccases for the polymerization of lignin to produce wood coating materials.
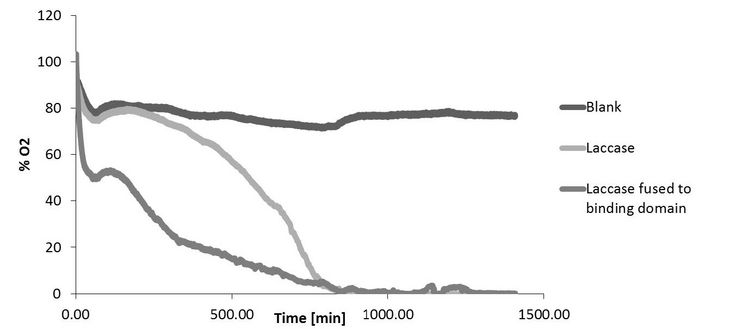
Figure 1: Oxygen consumption during lignin polymerization is increased by the use of a laccase fused to a substrate binding domain. 1 D. M. Mate and M. Alcalde, Microb. Biotechnol., 2017, 10, 1457–1467.
2 A. C. Mot and R. Silaghi-Dumitrescu, Biochem., 2012, 77, 1395–1407.
3 P. Varanasi, P. Singh, M. Auer, P. D. Adams, B. A. Simmons and S. Singh, Biotechnol. Biofuels, 2013, 6, 14.
4 A. Ortner, D. Huber, O. Haske-Cornelius, H. K. Weber, K. Hofer, W. Bauer, G. S. Nyanhongo and G. M. Guebitz, Process Biochem., 2015, 50, 1277–1283.
