UV detoxification and fermentation of spent liquor from pulp industry
SUPERVISOR: Georg GÜBITZ
PROJECT ASSIGNED TO: Tom DISTLER
At present, approximately 130 million tons of wood are used for the production of chemical pulp each year worldwide. The production process generates relatively large amounts of process spent liquor, most of which is currently used for thermal purposes. The spent liquors contain a wide range of degradation products of cellulose, hemicelluloses, lignin and other wood components. These include monosaccharides, sugar acids, organic acids, lignosulfonate salts, extractives and their degradation products. The monosaccharides of the waste lye provide an optimal starting substrate for a variety of biotechnological applications. With the help of suitable microorganisms, biofuels, animal feed, bio-based plastics and platform chemicals can be obtained from the sugars (Fig. 1) 1,2,3.
However, In addition to biotechnologically usable substances, the sulphite spent liquor contains inhibitors that have a negative effect on the metabolism of the microorganisms or may even be toxic to them. Potential inhibitors may include organic acids, phenols, furan derivatives, wood extractives and metallic cations. They prevent the direct fermentative use of spent liquors 4,5,6. Therefore, the complete removal of inhibiting substances is necessary for the process efficiency of the fermentation.
The idea of the project is to irradiate the sulphite spent liquor with UV light before fermentation, which should lead to dimerization and further polymerization of inhibiting phenol derivatives in the spent liquor. This reaction significantly increases the molar mass of these components in relation to the sugars, which makes them easier to separate. Alternatively, initiator polymers can be added for better control of the reactions, which promote the polymerization of the phenols in the waste lye (Fig. 2.)7.
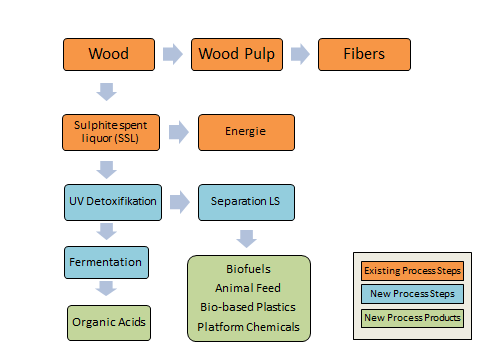
Figure 1: Existing fiber production process with proposed new process steps and new process products.
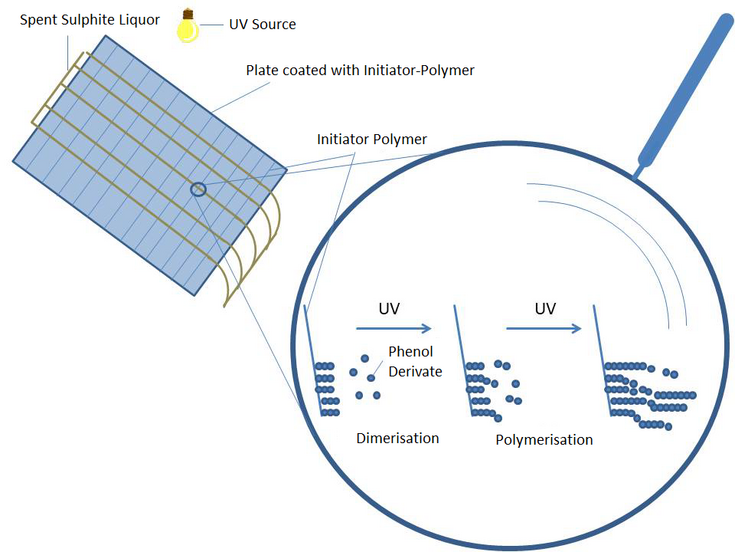
Figure 2: Possible Reactor configuration to polymerize Phenol Derivates in Spent Sulphite Liquor with UV Light
Literature
1 Sjöde, A.; Frölander, A.; Lersch, M.; Rodrud, G. (2011): Lignocellulosic Biomass Conversion
[PCT/EP2009/009046], 2011
2 Cheng, C.-L.; Lo, Y.-C.; Lee, K.-S.; Lee, D.-J.; Lin, C.-Y.; Chang, J.-S. (2011): Biohydrogen production
from lignocellulosic feedstock. In: Bioresource Technology 102 (18), S. 8514–8523.
3 Ren, N.-Q.; Zhao, L.; Chen, C.; Guo, W.-Q.; Cao, G.-L. (2016): A review on bioconversion of lignocellulosic biomass to H2: Key challenges and new insights. In: Bioresource Technology 215, S. 92–99.4 Xavier, A. M. R. B.; Correia, M. F.; Pereira, S. R.; Evtuguin, D. V. (2010): Second-generation bioethanol from eucalypt sulphite spent liquor. In: Bioresource Technology 101 (8), S. 2755–2761.
5 Klinke, H. B.; Thomsen, A. B.; Ahring, B. K. (2001): Potential inhibitors from wet oxidation of wheat straw and their effect on growth and ethanol production by Thermoanaerobacter mathranii. In: Applied Microbiology and Biotechnology 57 (5-6), S. 631–638.
6 Jönsson, L. J.; Martin, C. (2016): Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. In: Bioresource Technology 199, S. 103–112.
7 Behboodi-Sadabad, F.; Zhang, H.; Trouillet, V.; Welle., A.; Plumeré, N.; Levkin, P. (2017): UV Triggered Polymerization, Deposition, and Patterning of Plant Phenolic Compounds. In: Advanced Functional Materials 27, 1700127.
