Investigation of Lytic Polysaccharide Monooxygenases on Solid Substrates
SUPERVISOR: Roland LUDWIG
PROJECT ASSIGNED TO: Adonis YANOS
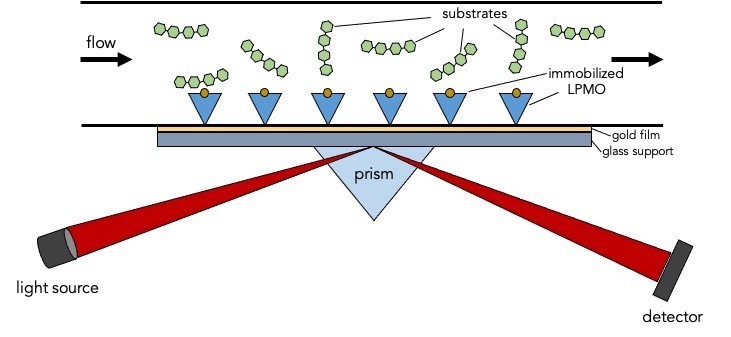
Lytic polysaccharide monooxygenases (LPMO) are enzymes capable of degrading solid and soluble recalcitrant polysaccharides such as cellulose, chitin, hemicelluloses and starch. This unique catalytic capability has inspired extensive research focusing on potential applications to produce value-added bioproducts. In order to fully utilize this enzyme, we need to understand its substrate-binding specificity and determine which factors affect its product formation. One way to study the enzyme-substrate interaction is through the use of surface plasmon resonance (SPR) techniques. Using SPR-based methods one can monitor the binding and interaction of LPMOs and its substrate in real-time. By immobilizing the enzyme onto the sensor chip, one can observe the affinity and specificity of LPMOs towards cellulose, chitin, and other insoluble as well as soluble carbohydrates. This will lead to the understanding of the enzyme’s substrate recognition and enzymatic specificity. SPR can also be used to measure the enzyme’s affinity for its substrate, as well as the rate of substrate binding and dissociation.
The research conducted within this doctoral thesis project aims to express and purify LPMO enzymes from various microbial sources. After which, these enzymes will be immobilized to a sensor chip and SPR techniques will be used to analyze the substrate binding affinity of LPMOs. Alternatively, sensor architectures mimicking natural biopolymers like amorphous or crystalline cellulose, hemicellulose or lignin fractions will be used in the SPR system to probe binding properties of the enzyme to different fractions of natural biomass. Furthermore, this study also aims to quantify catalytic turnover using amperometric sensor and other methods to detect LPMO products formed. By correlating binding affinity with catalytic efficiency, this study will provide a better understanding of LPMO functionality.