BREATH (Biofilm REsponsive Adjuvant as an alternative THerapeutic approach)
SUPERVISOR: Erik REIMHULT
PROJECT ASSIGNED TO: Goodness OSONDU-CHUKA
Bacteria within a biofilm are up to 1,000-fold more resistant to antibiotics and are inherently insensitive to the host immune response. This is particularly relevant for patients affected by Cystic Fibrosis (CF). Cystic fibrosis patients have a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which is responsible for the secretion of sticky mucus in the patient's airway, rendering them more vulnerable to infection. Indeed, once Pseudomonas aeruginosa colonizes the lungs, it can acquire a mucoid phenotype, which renders infection insensitive to antibiotics. Despite the intense development of anti-biotherapies, chronic lung infection in CF patients is extremely difficult or even impossible to treat, resulting in high mortality. The reasons for the extreme resistance of CF-Biofilm to drugs are not yet elucidated, but external stresses existing in the local environment of the lung along with the copious secretion of alginate in the biofilm have been suggested to play a crucial role in P. aeruginosa tolerance to antibiotics.
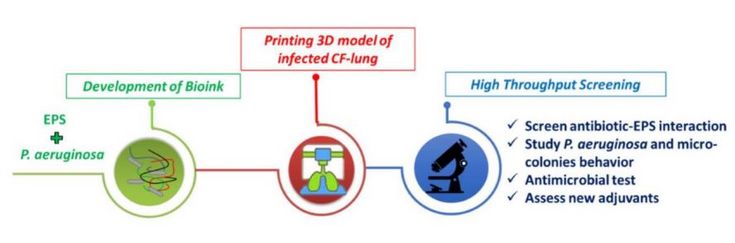
We hypothesize that by better understanding and then controlling how antibiotics interact with the main extra-cellular polymeric substances (EPS), we could improve existing therapies to eradicate microorganisms in mucoid biofilms. This hypothesis has not yet been investigated and could provide a breakthrough discovery on how microorganisms self-adapt to external cues. To validate our hypothesis, we will develop a 3D model obtained by bioprinting, aiming to reproduce essential features of CF-biofilm in a petri-dish, on which interplay between antibiotics and EPS can be studied using high-throughput screening (HTS). These model biofilms as well as in vitro biofilms of P. aeruginosa will be used to study the effect of antibiotics and adjuvants on the mechanical and structural properties of the EPS. The focus will be on the mechanistic understanding of binding partners and their effect on the distribution and efficacy of antibiotics on bacteria in biofilms.