Characterization of bacteria and biofilms by digital phase-contrast holography
SUPERVISOR: Erik REIMHULT
PROJECT ASSIGNED TO: Bhavana SATHISH
Healthcare-associated bacterial infections currently represent a high burden to the healthcare sector and patients worldwide. Further, bacteria and other biofilm-forming microorganisms pose an increasing health risk and economic burden as they easily contaminate everything from drinking water to food production and biorefineries. Still, detection of bacteria is a slow, manual, and costly process performed by manual cultivation on agar plates, microscopy, or sometimes PCR. Additionally, our ability to study and fight bacterial infections in the biofilm stage is still extremely limited.
In this project, we will explore digital phase-contrast holographic microscopy as a novel high-throughput method to identify not only bacterial species but also strains and bacteria in different growth stages. Our aim is to classify bacteria on their 3D-imaged colloidal properties, and eventually turn this method into an automated cytometry setup.
Additionally, bacteria and cells live in 3D environments that for bacteria are called biofilms and for cell tissue. There are few to no methods available to characterize the mechanical properties and the movement of bacteria and cells within these niches. Digital phase-contrast holographic microscopy combined with dynamic differential microscopy provides tools by which this could be possible. Therefore, we also aim to develop these methods, starting with hydrogels that model biofilms and wound dressings in contact with invasive bacteria.
Our overall aim is to provide new methods useful for diagnostics and wound dressing characterization within the H2020 MSC ITN project STIMULUS, which aims to create smart wound dressings to combat bacterial infections and antibiotic resistance in burn wounds care.
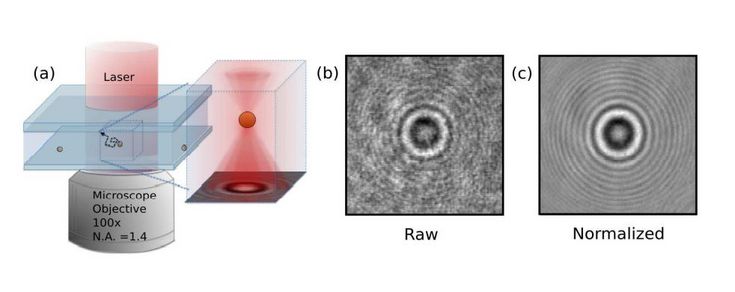
Fig.1. (a) In-line digital phase holographic microscope. A collimated laser beam illuminates the sample. Light scattered by the sample interferes with the unscattered portion of the beam. The interference pattern is recorded and then analyzed. (b) Unprocessed hologram recorded by the video camera. (c) The corresponding normalized hologram. (Cheong et al., Optics express 18.13 (2010): 13563-13573.)
Fig.2. Phase-contrast image of Staphylococcus aureus (Numerical reconstruction done from the holograms recorded using inline digital holography)
