Synthesis and evaluation of polysaccharide-based chiral stationary phases for enantiomer resolution
SUPERVISORS: Thomas ROSENAU, Hubert HETTEGGER
PROJECT ASSIGNED TO: Cuong Viet BUI
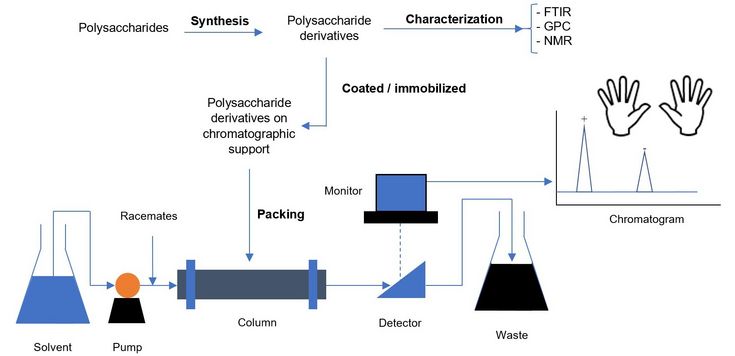
According to Louis Pasteur, the only well-marked line of demarcation between the chemistry of dead matter and the chemistry of living ones is based on asymmetric characteristics of naturally occurring organic compounds, which are present as enantiomers (chiral compounds, e.g. L-amino acids, D-sugars). Chirality is one basic feature in structure and chemistry of all living matter. Due to this asymmetric nature, chiral compounds exhibit different properties in biochemical systems. Enantiomers can be separated chromatographically (e.g. in HPLC setups) if the stationary phases themselves are chiral. Since Hesse and Hagel, the pioneers in successful application of polysaccharide derivatives as chiral stationary phases (CSPs based on cellulose triacetate) in 1976, different polysaccharides and their derivatives have been developed and used as CSPs due to their high separation power.
The purpose of this project is to exploit the different chemical nature of polysaccharides and their derivatives to generate polysaccharide-based CSPs with high enantioseparation power. The polysaccharide derivatives will be either coated on or covalently linked to silica as chromatographic support. The chiral separation performance and molecular recognition behavior of these materials will be evaluated by HPLC and other analytical techniques, including liquid- and solid-state NMR.
[1] Pasteur, L. C. R. Acad. Sci. 1858, 46, 615–618
[1] Hesse, G. and Hagel, R. Chromatographia 1973, 6, 277–280
