Advance Analytics of recombinant AAVs for gene therapy vectors
SUPERVISOR: Astrid DÜRAUER
PROJECT ASSIGNED TO: Sabrina LEIGHEB
Adeno-associated virus vectors are currently the most frequently used viral vectors for gene therapy. Since 2012, three AAV-based therapeutics have been approved, such as Zolgesma, Hemgenix and Luxturna (1). The development of advanced analytical techniques for the in-depth characterization of AAVs is essential to establish knowledge-based strategies for process development and optimization. Moreover, the establishment of analytical tools, is a crucial step to ensure the safety of the final product (2).
The major challenge in the AAvs production is the discrimination between functional particles, which correctly incorporated the Gene of Interest (GoI), and non-functional ones, which can be defined as empty, partially filled, overfilled particles and aggregates (3). The current state of the art in the discrimination and quantification of empty and filled AAVs is by Analytical Ultracentrifugation (AUC) which presents several drawbacks and therefore, the establishment of high-throughput innovative analytical tools is essential for a rapid determination of the empty-full ratio (4).
In this doctoral study, along with the establishment of the conventional techniques for AAVs and impurities characterization, such as ELISA, qPCR, Bradford and Picogreen Assay and SEC-UV, innovative methods for the in-depth characterization of rAAVs based on capillary electrophoresis and Taylor Dispersion Analysis, Field Flow Fractionation and multidimensional chromatography in combination with mass spectrometry will be developed.
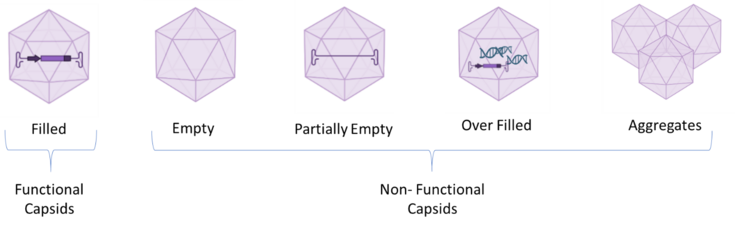
Figure 1 Schematic Representation of functional (Filled) and Non-Functional AAV capsids (Empty, Partially Empty, Overfilled, Aggregates)
References:
1. Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18(5):358-78.
2. Gimpel AL, Katsikis G, Sha S, Maloney AJ, Hong MS, Nguyen TNT, et al. Analytical methods for process and product characterization of recombinant adeno-associated virus-based gene therapies. Mol Ther Methods Clin Dev. 2021;20:740-54.
3. Green EA, Lee KH. Analytical methods to characterize recombinant adeno-associated virus vectors and the benefit of standardization and reference materials. Curr Opin Biotechnol. 2021;71:65-76.
4. Escandell JM, Pais DA, Carvalho SB, Vincent K, Gomes-Alves P, Alves PM. Leveraging rAAV bioprocess understanding and next generation bioanalytics development. Curr Opin Biotechnol. 2022;74:271-7.
