Process optimization for the biosynthesis of non-canonical amino acids
SUPERVISOR: Gerald STRIEDNER
PROJECT ASSIGNED TO: Claudia LACOMBE
Non-canonical amino-acids (ncAA) are valuable assets to expand the genetic code. Their diverse side-chain moieties can be used to introduce structural, chemical or functional modification in proteins.[1] They are incorporated into proteins using either site- or residue-specific incorporation (Figure 1). The residue-specific incorporation is based on the substrate tolerance of the host aminoacyl-tRNA synthetase for analogs of its specific canonical amino-acid. Under tightly controlled conditions, that is very low intracellular levels of this canonical amino-acid, the residue-specific incorporation procedure leads to the global replacement of all its occurrences in the target protein by the analog. For the site-specific incorporation, ncAAs are incorporated in response to an amber codon. Bio-orthogonal reactive ncAAs are of particular interest to extend the classical methods for protein modification. Orthogonal translation systems consisting of a pair of aminoacyl-tRNA synthetase and an amber suppressor tRNACUA have already been devised for lysine and phenylalanine derivatives carrying bio-orthogonal azide- and alkyne-side chains.[2]
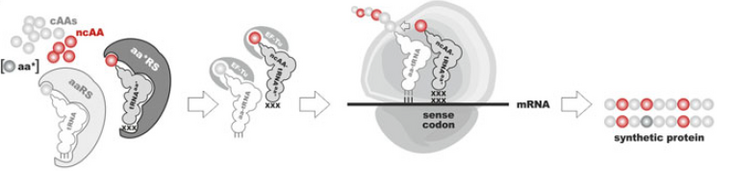
Figure 1: Incorporation of non-canonical amino-acids, reproduced from (Wiltschi, 2016)[1]
While site-specific incorporation is the method of choice if the target protein contains several essential residues to exchange with an analog, the incorporation efficiency is lower than with the residue-specific incorporation.[3] In a word, residue- and site-specific incorporation of ncAA are complementary methods and valuable tools to diversify the chemical modification of proteins. Due to their high cost, ncAAs require efficient and improved routes of production. The ncAAs’ chemical synthesis is limited by the hazardous reagents and waste products as well as the labor-consuming production.[4] The biotechnological approach is currently also limited by the reactions and substrate scope to biocatalyze ncAAs production.[4] This approach must be advanced to reach an efficient, green and cost-effective production of ncAA using inexpensive precursors. To this end, the project’s goal is to devise an optimized bioprocess for an ncAA’s biotechnological production in bioreactors cultures.
[1] B. Wiltschi, Protein building blocks and the expansion of the genetic code, in Synthetic Biology, A. Glieder, C. P. Kubicek, D. Mattanovich, B. Wiltschi, M. Sauer, Editors. 2016, Springer International Publishing: Cham. p. 143.
[2] A. Dumas, L. Lercher, C. D. Spicer, B. G. Davis, Chem. Sci. 2015, 6, 50.
[3] F. Tobola, E. Sylvander, C. Gafko, B. Wiltschi, Interface Focus 2019, 9, 20180072.
[4] P. J. Almhjell, C. E. Boville, F. H. Arnold, Chemical Society reviews 2018, 47, 8980.
