Downstream development for an integrated continuous manufacturing process for monoclonal antibodies
SUPERVISOR: Alois JUNGBAUER
PROJECT ASSIGNED TO: Gabriele RECANATI
As biopharmaceutical industries are moving towards continuous biomanufacturing, the development of downstream platform processes able to operate in fully continuous mode gained a great interest of both researchers and industrial partners in recent years.
Perfusion processes reported in the literature are today characterized by a high titer ranging from 3 to 15 g/L, accompanied by high levels of impurities (depending on the harvest method). Both these represent a challenge for the state of the art capture and polishing steps, usually carried on by chromatographic techniques. As an alternative, precipitation of the recombinant protein was shown to be feasible and effective as capture method for continuous downstream operation. The mAb co-precipitates with some of the impurities present in the crude supernatant and can then be concentrated 10/20-fold and washed through tangential flow filtration in a multi-stage feed-and-bleed configuration. The subsequent step is the redissolution of the precipitate in a defined buffer system at low pH, which is then hold at low pH for the first step of viral inactivation. Afterwards, a pH adjustment step is needed and the product is finally directed to the polishing unit. All these steps shall be optimized by screening of critical parameters for impurities removal.
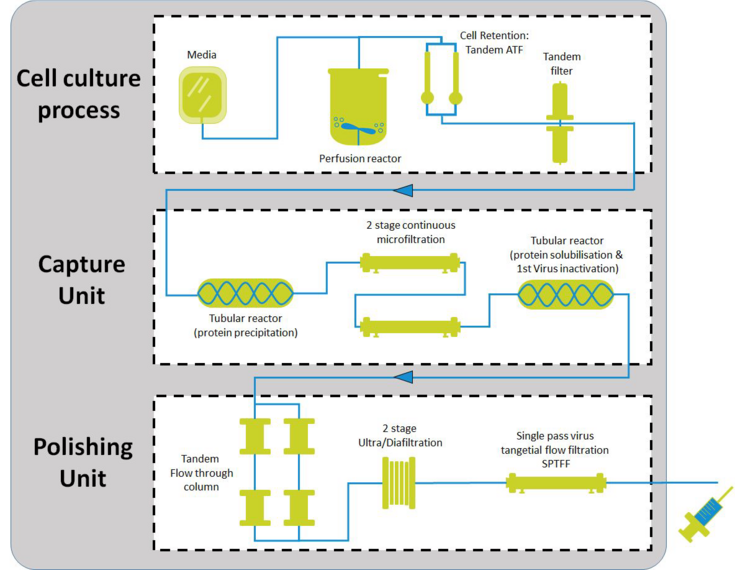
This PhD thesis is part of a research project funded by Bilfinger Industrietechnik Salzburg GmbH and the Austrian Research Promotion Agency (FFG) to establish a fully automated, integrated and continuous end-to-end platform process skid for the production of biopharmaceuticals. Herein, a continuous process will be established in three unit operations. In this research project, the skid of a membrane-based capture and polishing steps where the entire process is controlled, should be drawn. Using a custom-made set-up, a capture process will be established and set-up on lab scale experiments. Its feasibility for full scale (pilot) manufacturing will be evaluated and tested using different membrane geometries and process parameters (such as initial concentration and impurities level, concentration factor, recirculation flow rate and so on) .
Furthermore the link to the perfusion and polishing units with regard to integrated processing, by establishment of process models for monitoring and control purposes as well as the implementation, testing of robustness and usability of developed control algorithms will be examined in more detail. By implementation of the designed control concepts a final stress and robustness testing of the entire end-to-end prototype in a fully continuous and automated manufacturing process will show its applicability and proof of concept of this study.
