Process development for high yield fermentation of active recombinant lectins expressed in Escherichia coli
SUPERVISOR: Gerald STRIEDNER
PROJECT ASSIGNED TO: Natalia DANIELEWICZ
Protein-carbohydrates interactions pave the way for many pathogens and pathogen-like entries inside the eukaryotic systems. Such sugar-binding lectins provide means for finding novel cancer treatment, vaccine strategies, protein-based therapeutics and pest control applications. Here, we present interdisciplinary research combining directional secretion of carbohydrate specific protein in enGenes-X-press E.coli propagated by novel purification process enabling cumbersome constructs to be free of the soluble tags.
For that purpose, we propose dual approach where cleavable Tobacco Etch Virus (TEV) protease will be fused to solubility enhancer and periplasmic exporter, protein A. This secretion tag counters protein aggregation, N-terminal degradation, allows a straightforward affinity purification via an IgG column and is suiting for the enGenes X-press strain that specializes in uncoupled growth in E. coli while progressively leads to high yield protein production1, 2. Such TEV-protein A fusion will cohere with expression of the carbohydrate-specific target lectin, often fused to TEV- cleavable Mannose Binding Protein (MBP), that is often produced in insoluble form. For example, we intend to separately produce in enGenes- X- press E.coli strain recombinant TEV-protein A and non-toxic A2-CTB Vibrio Cholera fused to MBP. By design, the final periplasmic and cell supernatants extracts combined from both fermentation should lead to TEV- guided cleaving reaction, leaving A2-CTB target ready for single step purification. Alternatively, we shall follow dual expression in a single cell under differently induced promoters.
We intend to continue with our innovative approach by designing complementary manufacturing process composed of coupled downstream and upstream process enabling on-demand production of lectin with soluble tags3 (Fig.1.). The system will consists of two independently running chemostats enGene-X-press strain bioreactor that can bear high yield continuous production of the carbohydrate-specific proteins with allosterically oriented solubility tag and cleaving appliance, such as TEV protease, selective for the tag. Filtered and cell separated supernatants containing extracellularly expressed target biomolecules will then mix and the carbohydrate-specific protein is cleaved off its soluble partner and undergoes multi-stage fluidically- connected chromatography with integrated sensors and system controllers. Furthermore, we intend to integrate analytical quality assessment module sequestered after the purification, evaluating specificity, affinity and stability of the final lectin product.
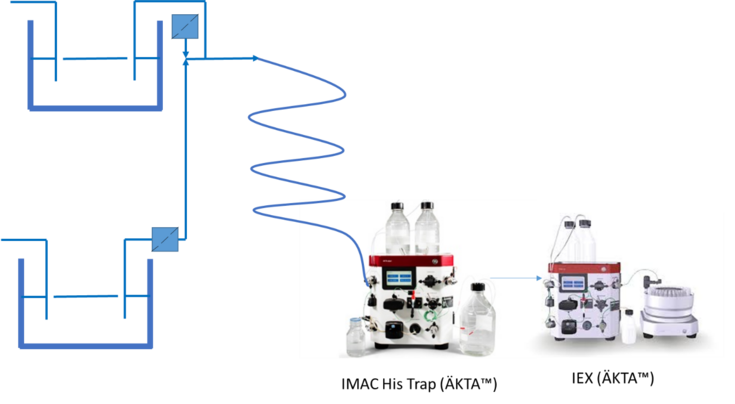
Fig.1. In parallel steady-state fermentation process of a carbohydrate-specific protein and a protease with solubility tag specificity. Operational production system (upstream) is coupled to purification module (downstream). The system is suitable for enGenes-X-press strain with a cell supernatants high yield protein expression. The fermentation solution, containing separately target carbohydrate- binding protein and the protease, flow through the membranes that execute the cell/supernatants separation. The perfusates mix and throughout fluidic-based system progress to the purification module composed of the Immobilized metal affinity chromatography (IMAC) and Ion-exchange chromatography (IEX).
Slightly different approach will be executed for carbohydrate- specific protein that folding and active conformation is strictly depended on disulfide bridging maintained by thiol groups. Production of correctly folded disulfide- rich lectins can be independent of the most exquisite solubility tags implemented during the expression phase. Thereof, we intend to re-engineer the enGenes-X-press strain with diminished cytoplasmic reductive pathways, consequently, allowing for formation of disulfide bonds4. Our technology will combine ECBD_2707 gene suppression (thioredoxin reductase of strain B), chromosomal integration of the disulfide bond isomerase, DsbC, and co-expression of mutant thioredoxin and PDI homologs for improved yields of disulfide- rich lectins. Production of such demanding lectins will be conducted via cytoplasmic expression followed by purification via cell lysis sonication.
1. Heel, T., Paal, M., Schneider, R. and Auer, B. (2010). Dissection of an old protein reveals a novel application: domain D of Staphylococcus aureus Protein A (sSpAD) as a secretion - tag. Microbial Cell Factories, 9(1), p.92.
2. Mairhofer, J, Striedner, G, Grabherr, R, & Wilde, M (2016). Patent No.WO2016/174195A1. Uncoupling growth and protein production
3. Crowell, L., Lu, A., Love, K., Stockdale, A., Timmick, S., Wu, D., Wang, Y., Doherty, W., Bonnyman, A., Vecchiarello, N., Goodwine, C., Bradbury, L., Brady, J., Clark, J., Colant, N., Cvetkovic, A., Dalvie, N., Liu, D., Liu, Y., Mascarenhas, C., Matthews, C., Mozdzierz, N., Shah, K., Wu, S., Hancock, W., Braatz, R., Cramer, S. and Love, J. (2018). On-demand manufacturing of clinical-quality biopharmaceuticals. Nature Biotechnology, 36(10), pp.988-995.
4. Lobstein, J., Emrich, C., Jeans, C., Faulkner, M., Riggs, P. and Berkmen, M. (2012). SHuffle, a novel Escherichia coli protein expression strain capable of correctly folding disulfide bonded proteins in its cytoplasm. Microbial Cell Factories, 11(1).
