Cell engineering for AAV production
SUPERVISOR: Astrid DÜRAUER
PROJECT ASSIGNED TO: Maria TOTH
The adeno-associated virus (AAV) is a small (app. 25 nm), non-enveloped virus that carries a ssDNA genome of 4.7 kb. AAVs depend on coinfection with helperviruses such as Adenovirus (AdV) or Herpes Simplex Virus (HSV) for replication. Due to non-pathogenicity of AAVs, they are a suitable vectors for gene therapy. AAVs deliver their viral genome into different tissues depending on the serotype and persist in cells lifelong [1]. Several AAV gene therapies such as Hemgenix, Luxturna or Zolgensma have been approved by EMA, demonstrating the feasibility of AAVs as gene therapy vectors. In the current state-of-the-art production process, three plasmids are co-transfected into mammalian cells: one expressing the replicase (Rep) and the three capsid proteins (Cap), the second expressing all essential helper factors from Adenovirus (AdV) and the third bearing the target gene. AdV helper factors E1B, E2A, E4 and VA are essential for the replication and efficient production of AAVs in mammalian cells [2]. The production of required amounts of these plasmids is costly and labour-intensive. The triple transfection depends on various parameters and is currently not efficient. To address this problem, cell lines that stably express Rep/Cap and AdV helper proteins were designed in many variations [3-7]. This approach improves process scalability and reduces the number of plasmids required. The limitation here so far was the toxicity of some of these proteins when constitutively produced. This reduces the ability to cultivate and expand cells efficiently to achieve high cell numbers for transfection, a prerequisite for high yields.
Inducible systems would enable cells to grow unrestrained during expansion and allow for expression of the toxic proteins only during the production of gene therapy vectors. Tuneable expression systems would have the added advantage that fine-tuned optimization of expression levels of the different proteins and their combinations for most efficient virus production becomes possible. Further, these cell lines can be used to test other optimization strategies with a given background of viral gene expression levels.
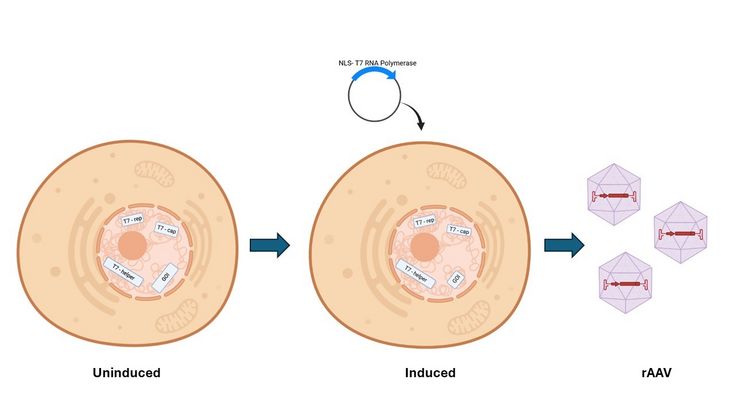
We will generate and characterize different packaging and producer cell lines that contain Rep/Cap expressing genes and AdV helper protein expressing genes (E4, E2a and VA) in different combinations integrated into their genome under control of inducible and/or tuneable promoters. To achieve a reproducible genomic background, the genes will be transferred into the genome of the producer cell line by site directed integration, to avoid any background diversity in behaviour that is due to the integration site rather than the recombinant gene or its expression level. The cassettes integrated will contain Rep, Cap or AdV helper proteins and different combinations thereof. In the first experiments, non-integrated will be complemented by transient transfection with a plasmid. To reduce the inherent toxicity of Rep, Cap and E2A, different inducible promoters will be tested, ideally resulting in a system that enables dose depending inducibility, so that different expression levels and their combinations can also be characterized. If it turns out that stable integration of Rep and Cap is advantageous, then several cell lines can be established since based on the site-directed integration, different variants of Rep and Cap can be efficiently exchanged in a comparable system, so as to rapidly determine optimal combinations and expression levels.
[1] Meier AF, Fraefel C, Seyffert M. The Interplay between Adeno-Associated Virus and its Helper Viruses. Viruses. 2020 Jun 19;12(6):662. doi: 10.3390/v12060662. PMID: 32575422; PMCID: PMC7354565.
[2] Geoffroy, M.C. & Salvetti, A. Helper functions required for wild type and recombinant adeno-associated virus growth. Curr Gene Ther. 5, 265-271. doi: 210.2174/1566523054064977. (2005).
[3] Gao, G.P. et al. High-titer adeno-associated viral vectors from a Rep/Cap cell line and hybrid shuttle virus. Hum Gene Ther. 9, 2353-2362. doi: 2310.1089/hum.1998.2359.2316-2353. (1998).
[4] Chadeuf, G. et al. Efficient recombinant adeno-associated virus production by a stable rep-cap HeLa cell line correlates with adenovirusinduced amplification of the integrated rep-cap genome. J Gene Med. 2, 260-268. doi: 210.1002/1521- 2254(200007/200008)200002:200004200003.200000.CO;200002-200008. (2000).
[5] Qiao, C., Wang, B., Zhu, X., Li, J. & Xiao, X. A novel gene expression control system and its use in stable, high-titer 293 cell-based adenoassociated virus packaging cell lines. J Virol. 76, 13015- 13027. doi: 13010.11128/jvi.13076.13024.13015-13027.12002. (2002).
[6] Gao, G.P. et al. Rep/Cap gene amplification and high-yield production of AAV in an A549 cell line expressing Rep/Cap. Mol Ther. 5, 644- 649. doi: 610.1006/mthe.2001.0591. (2002).
[7] Clark, K.R., Voulgaropoulou, F., Fraley, D.M. & Johnson, P.R. Cell lines for the production of recombinant adeno-associated virus. Hum Gene Ther. 6, 1329-1341. doi: 1310.1089/hum.1995.1326.1310-1329. (1995)
