MISTER - Metabolic lncorporation of latent fast reacting thioeSTERs
SUPERVISOR: Gerald STRIEDNER
PROJECT ASSIGNED TO: Arshia ARASTEH KANI
The site specific incorporation of more than 200 non canonical amino acids (ncAA) into proteins has unlocked unprecedented possibilities for directed protein modification.1 NcAAs containing reactive side chain moieties are of particular interest because they enable the controlled functionalization of proteins with, e.g. drugs, fluorophores, DNA and RNA, lipids or other proteins and peptides using biorthogonal chemistry (‘click chemistry’).2 To install ncAAs with bioorthogonal side chain chemistry at a pre defined position in a protein, an engineered aminoacyl tRNA synthetase (aaRS) and a cognate tRNA are required. 3 (Figure 1). These so-called orthogonal pairs can be evolved using a modified E. coli strain as a screening system, which employs different reporters to assess the incorporation of an ncAA while canonical amino acids are not accepted.
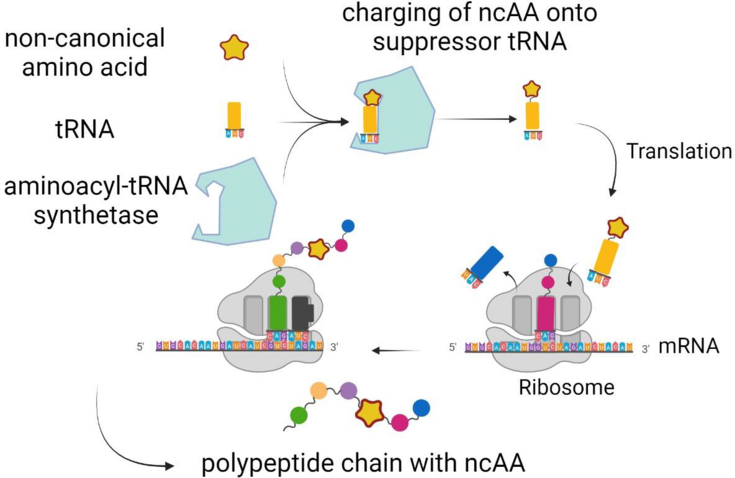
Figure 1: Site specific incorporation of an ncAA at an in-frame amber stop codon.
In this project, oxoSEAK, which is a lysine derivative is going to be used as a reactive ncAA. This ncAA carries an oxalyl amide as latent thioester in its side chain, which has a high potential for peptide and protein modification using click chemistry4. In a previous study, peptides labeled chemically with oxalyl bis(2-sulfanylethyl)amido (oxoSEA) latent thioester precursors crosslinked with peptides containing an N-terminal cysteine at exceptional speed and with excellent chemoselecitivity. During this native chemical ligation (NCL) reaction, peptide conjugates with a stable oxalamide crosslink were formed. The reaction proceeded under mild conditions in dilute as well as complex aqueous media, e.g. buffers and cell lysate4. To advance oxoSEA-NCL to the next step, the reactive oxoSEA moiety in proteins will be installed by genetic incorporation.
