Functionalised non-woven nanofibers for the purification of Virus-like particles (VLPs) in Biotechnological Downstream Processing
SUPERVISOR: Alois JUNGBAUER
PROJECT ASSIGNED TO: Alexander ZOLLNER
Production of bionanoparticles such as viruses, virus-like-particles or extracellular vesicles is a quickly developing field in modern bioprocess engineering. These particles build a promising new platform for upcoming new approaches in biopharmaceutical drug design and medical treatments in regards to e.g. new generation vaccines and gene therapies [1]–[3].
Due to their highly complex structure (Figure 1), compared to more established products like proteins or antibodies, purification and analytics of bionanoparticles produced in biotechnological processes are still a demanding challenge. Current downstream processing strategies are based on chromatographic processes using different types of media and separation principles (e.g. size exclusion, ion exchange, affinity chromatography, Figure 2). Product quantity and quality determination require the combination of well-established analytical methods focusing on nanoparticles like Nanoparticle Tracking Analysis (NTA), Multi-Angle Light Scattering (MALS) and Cryo-Electron Microscopy (cryo-EM) along with standard biochemical methods like SDS-PAGE, Western Blot and DNA and protein quantitation assays [2]–[7].
The purification methods used are generally time and material consuming and often do not provide the purity required for medical application. Thus, an encouraging new approach is the use of membranes functionalized with affinity ligands as a powerful separation tool, which would allow product capture and purification in a reduced number of steps, significantly reducing process time and material costs.
The goal of this project is to establish the use of non-woven nanofiber membranes for the capture and purification of bionanoparticles such as viruses and virus-like particles. The membranes will be functionalized with affinity ligands in order to specifically bind the desired bionanoparticles and efficiently separate them from any process- and product-related impurities like host cell proteins, chromatin, vesicles and other bionanoparticles produced by the cells. This strategy should finally enable a switch from resin-based chromatography to a one-step membrane-based purification process resulting in highly pure bionanoparticles ready for vaccine production or gene therapy applications.
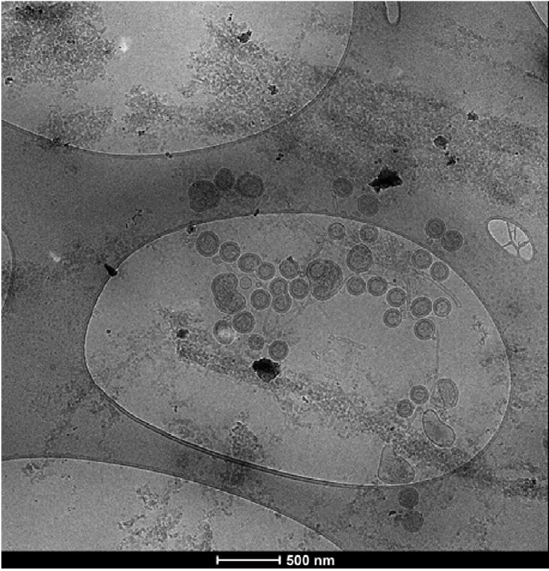
Figure 1: Cryo-TEM image showing HIV-1 gag VLPs and several host cell derived particles [8].
Figure 2: Representative chromatogram of a resin-based purification of influenza VLPs using affinity chromatography [3].
[1] T. Vicente, A. Roldão, C. Peixoto, M. J. T. Carrondo, and P. M. Alves, “Large-scale production and purification of VLP-based vaccines.,” J. Invertebr. Pathol., vol. 107 Suppl, pp. S42-8, Jul. 2011, doi: 10.1016/j.jip.2011.05.004.
[2] T. M. Lima, M. O. Souza, and L. R. Castilho, “Purification of flavivirus VLPs by a two-step chomatographic process.,” Vaccine, vol. 37, no. 47, pp. 7061–7069, Nov. 2019, doi: 10.1016/j.vaccine.2019.05.066.
[3] M. G. Moleirinho et al., “Baculovirus affinity removal in viral-based bioprocesses,” Sep. Purif. Technol., vol. 241, p. 116693, 2020.
[4] P. Steppert et al., “Purification of HIV-1 gag virus-like particles and separation of other extracellular particles.,” J. Chromatogr. A, vol. 1455, pp. 93–101, Jul. 2016, doi: 10.1016/j.chroma.2016.05.053.
[5] K. Reiter, P. Pereira Aguilar, D. Grammelhofer, J. Joseph, P. Steppert, and A. Jungbauer, “Separation of influenza virus-like particles from baculovirus by polymer-grafted anion exchanger.,” J. Sep. Sci., vol. 43, no. 12, pp. 2270–2278, Jun. 2020, doi: 10.1002/jssc.201901215.
[6] T. Koho et al., “Purification of norovirus-like particles (VLPs) by ion exchange chromatography.,” J. Virol. Methods, vol. 181, no. 1, pp. 6–11, Apr. 2012, doi: 10.1016/j.jviromet.2012.01.003.
[7] P. Pereira Aguilar et al., “Polymer-grafted chromatography media for the purification of enveloped virus-like particles, exemplified with HIV-1 gag VLP.,” Vaccine, vol. 37, no. 47, pp. 7070–7080, Nov. 2019, doi: 10.1016/j.vaccine.2019.07.001.
[8] P. Pereira Aguilar et al., “Capture and purification of Human Immunodeficiency Virus-1 virus-like particles: Convective media vs porous beads.,” J. Chromatogr. A, vol. 1627, p. 461378, Sep. 2020, doi: 10.1016/j.chroma.2020.461378.
