Model based process development and scale up of primary recovery for biopharmaceutical production
SUPERVISORS: Rainer HAHN, Astrid DÜRAUER
PROJECT ASSIGNED TO: Markus BERG
Since ancient times bioactive compounds are widely used as a source of medication to promote good health. Therefore, every year a lot of chemically or biologically produced active compounds are required. For instance, in 2019 about 48 new recombinantly and non-recombinantly produced biopharmaceuticals had been approved by FDA1.
The current state of the art cascade for implementing a production process in manufacturing scale of such a biotherapeutic is based on a lot of single steps and experiments, which are time and material consuming. Even more tons of material and equipment would be mandatory for successfully accomplishing and evaluating large scale experiments. To reduce costs, initial and key experiments necessary for setting up the process are usually performed on micro and laboratory scale. The gained knowledge is then used to allow a stepwise scale up of parameters and process steps, which furthermore is related to optimization, verification and analysis. Finally, after an extensive amount of experiments and intermediate steps manufacturing scale is reached and ideally the product is ready for production (Figure 1).
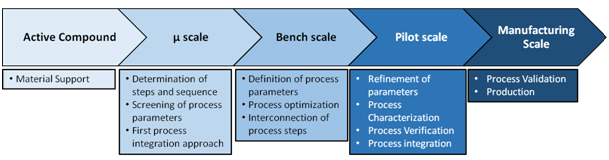
Figure 1: State of the art process of upscaling
Within this up-scaling progress some fundamental challenges have to be overcome, which are more than significant and decisive for a well-working production process. Due to technical limitations and differences between micro/laboratory and production scale each unit operation has to be adapted to the specific scale and furthermore optimized. Moreover, these technical limitations can result in a different physical mechanism within a specific unit operation and this mainly leads to a change of parameters and possibly in an alteration of yield and quality of the process step as well.
Therefore, numerous experiments have to be performed to gain knowledge on the interrelation of the input and output parameters within each unit operation on each scale and of course on the interrelation within the entire process on each scale. Major questions that have to be answered are for instance:
how does a change of a specific parameter influences the unit operation yield and overall production yield?
In this doctoral thesis a strategy/model will be generated, which allows a direct and relatively fast scaling of downstream processes in both directions.
Basically, the project is divided into different tasks with pre-defined goals and content. All results and information gained within each task should ideally help to build up preferably mechanistic or statistical model, which enables up- and downscaling with a reduced number of intermediate steps and related experiments.
Additionally, another main focus of the thesis is the evaluation and prediction of chromatographic behavior across the scales.
References:
- https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019 (accessed on 29.07.2020, 09:20).
