Next generation flocculants for intensified monoclonal antibody production
SUPERVISOR: Rainer HAHN
PROJECT ASSIGNED TO: Anna FRANK
Monoclonal antibodies are still the most dominant biopharmaceutical regarding market approvals as well as sales in the last years. To increase the monoclonal antibody titers, cells are cultivated at high cell densities and harvest is performed at low viabilities. With this cultivation strategy however, a higher content of solid biomass composed of cells and cell debris as well as a higher concentration of host cell proteins and host cell DNA, must be removed. This increases the burden on the subsequent clarification unit operations. In traditional dead-end filtration, filter capacities are consequently significantly lower for high titer processes leading to a higher requirement in filter area and thus higher costs. Also, the levels of soluble host cell derived impurities such as host cell DNA and host cell proteins are higher since more cells are lysed at the time of harvest.
In the course of this project the selected flocculants (cationic polymers) having different physio-chemical properties are tested for their ability to flocculate monoclonal antibody producing mammalian cells and additional impurities. Cell flocculation during primary recovery facilitates separation of cells by centrifugation or depth filtration leading to a reduction in necessary filter area or a faster centrifugation process. Additionally, negatively charged host cell derived impurities such as host cell DNA and host cell proteins could be removed during flocculation, which is the main goal of this work. This will lead to a lower burden for the complete purification train and thus a reduction or even omission of some unit operations. The performance of the tested flocculants is evaluated by their filtration behavior in depth filtration as well as by analytical methods to determine the remaining impurities after flocculation.
The developed intensified process will we compared to the conventional manufacturing train of a monoclonal antibody product.
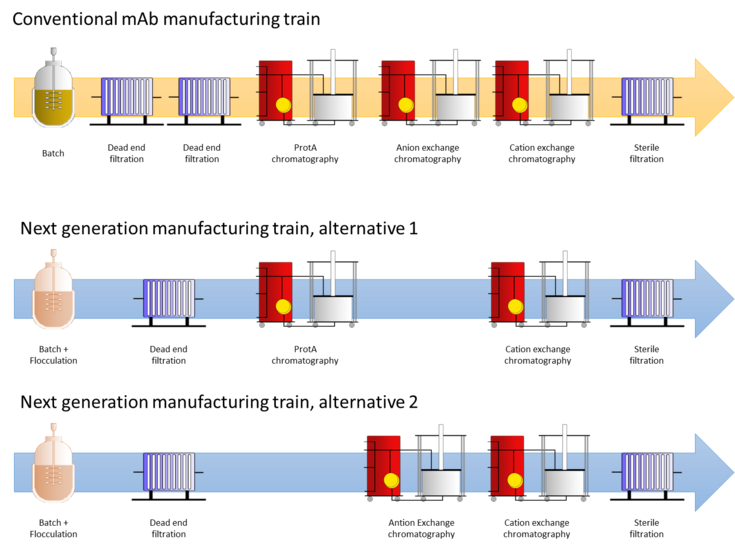
Figure 1: Graphic abstract - Reduction of unit operations in a conventional monoclonal antibody (mAb) manufacturing train by addition of next generation flocculants
