Residence time distribution (RTD): effect on start-up and shut-down for product collection
SUPERVISOR: Alois JUNGBAUER
PROJECT ASSIGNED TO: Narges LALI
Today, Biopharmaceutical industry and regulatory authorities are highly interested to establish integrated continuous biomanufacturing processes because they expect reduced costs, an improved economic footprint, an improved or more consistent quality, but they face many challenges [1,2]. An important topic in the continuous processing is the residence time distribution (RTD), showing how the material is moving through the process. For this purpose, we need to characterize the RTD of all involved individual unit operations and the interconnected setup of multiple unit operations [3].
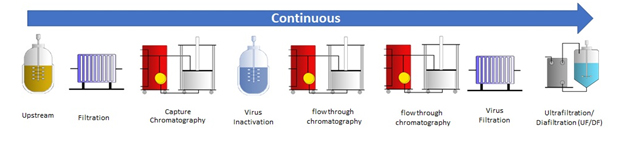
Continuous upstream and downstream process
The goal of the research project is establishing a model for the residence time distribution of a complete continuous downstream process for optimizing the start-up and shut-down times as well as where to cut for product collection for continuous processes. This is crucial for understanding and managing possible process disturbances as well as the start and end of the process. To achieve a complete model for the whole downstream chain, each unit operation will be modelled individually before combining all models into a complete process model. The unit operations in this research project will be based on an existing scheme for antibody purification.
The major accomplishments expected in this project are:
- Establishing a program that can handle multiple residence time distributions and the combination into a process scheme with dynamic sequence
- Establish the model descriptions for each individual unit operation in the process chain of a monoclonal antibody purification
- Verifying the model assumptions in terms residence time distribution experimentally for each unit operation.
References
[1] Cataldo, A.L., Burgstaller, D., Hribar, G., Jungbauer, A., Satzer, P. Economics and ecology: Modelling of continuous primary recovery and capture scenarios for recombinant antibody production (2020) Journal of Biotechnology, 308, pp. 87-95.
[2] Konstantinov, K. B., & Cooney, C. L. (2015). White paper on continuous bioprocessing. May 20–21, 2014 continuous manufacturing symposium. Journal of pharmaceutical sciences, 104(3), 813-820.
[3] GUIDANCE DOCUMENT Quality Considerations for Continuous Manufacturing Issued by: Office of Medical Products and Tobacco, Center for Drug Evaluation and Research document Nr. FDA-2019-D-0298 February 2019
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 812909 CODOBIO, within the Marie Skłodowska-Curie European Training Networks framework.
