Virus-like particle production in insect cells
SUPERVISOR: Alois JUNGBAUER
PROJECT ASSIGNED TO: Lena ACHLEITNER
The baculovirus expression vector system, in combination with insect cells as hosts, is widely used to produce recombinant proteins1, viruses and virus-like particles (VLPs) for research, human and veterinary applications2. This system has been available and thoroughly investigated since the 1960´s and it has been found that some insect cell lines in use have a persistent adventitious viral infection and are therefore associated with a potential biosafety hazard3. At the “Austrian centre of industrial biotechnology” (acib) and at the “University of Natural Resources and Life Sciences” (BOKU), we work with a virus-free cell line derived from the commercially available HighFiveTM cell line, called Tnms423. It was shown in the past, that this cell line can produce VLPs consistently with the baculovirus expression vector system4–6 (Figure 1) and is equally well suited as HighFiveTM cells4. Although the baculovirus expression vector system has high expression rates due to a strong promoter, it not only allows the production of VLPs but also of baculovirus. The two particles are similar in size and have overlapping surface proteins, which makes them difficult to distinguish and separate and consequently challenging to quantify. Alternatives like transient transfection using plasmids7 will therefore be investigated.
The cultivations in our laboratory have been and are still carried out mostly in shaking flask cultures. To overcome the limits of scalability, a bioreactor platform process will be developed to produce VLPs in Tnms42 cells at lab scale and ready to be scaled up to pilot or production scale. Therefore, cell culture conditions and infection parameters, such as time of infection, multiplicity of infection, time of harvest and others will be investigated for titre optimisation and reduction of by-products and impurities. In this project, a high throughput method for monitoring the production will be developed to simplify analytics and accelerate process optimisation. Accordingly, a well-suited model product with unique characteristics to distinguish from other particle population, but similar to a real product, is needed. Finally, an integrated capture step at the end of production will be implemented for product capture and/or purification without intermediate steps, such as cell separation and storage.
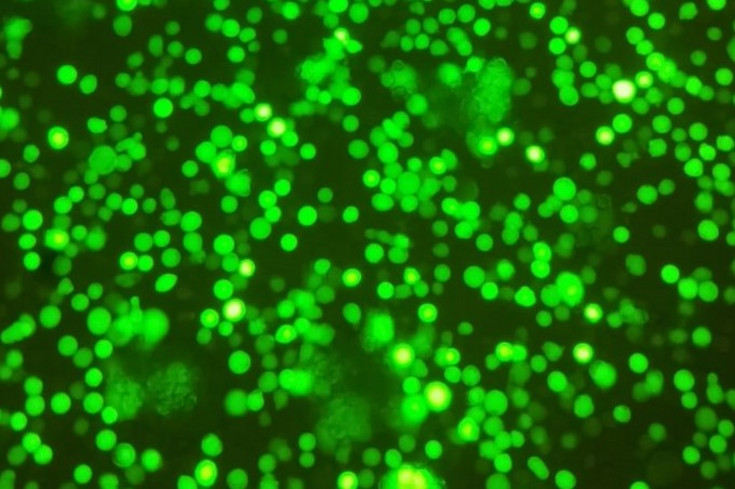
Figure 1: Tnms42 infected with baculo virus expressing yellow fluorescent protein and gag VLP
1. Bédard, C., Kamen, A., Tom, R. & Massie, B. Maximization of recombinant protein yield in the insect cell/baculovirus system by one-time addition of nutrients to high-density batch cultures. Cytotechnology 15, 129–138 (1994).
2. Tapia, F., Vázquez-Ramírez, D., Genzel, Y. & Reichl, U. Bioreactors for high cell density and continuous multi-stage cultivations: options for process intensification in cell culture-based viral vaccine production. Appl. Microbiol. Biotechnol. 100, 2121–2132 (2016).
3. Geisler, C. & Jarvis, D. L. Adventitious viruses in insect cell lines used for recombinant protein expression. Protein Expr Purif. 144, 25–32 (2018).
4. Klausberger, M. et al. Off-target effects of an insect cell-expressed influenza HA-pseudotyped Gag-VLP preparation in limiting postinfluenza Staphylococcus aureus infections. Vaccine 38, 859–867 (2020).
5. Strobl, F., Duerkop, M., Palmberger, D. & Striedner, G. High shear resistance of insect cells: the basis for substantial improvements in cell culture process design. Sci. Rep. 11, 9413 (2021).
6. Strobl, F., Ghorbanpour, S. M., Palmberger, D. & Striedner, G. Evaluation of screening platforms for virus-like particle production with the baculovirus expression vector system in insect cells. Sci. Rep. 10, 1–9 (2020).
7. Puente-massaguer, E. et al. PEI-Mediated Transient Transfection of High Five Cells at Bioreactor Scale for HIV-1 VLP Production. Nanomaterials 10, (2020).
