Omics-Driven Optimisation of CQAs in Industrial CHO Fed-Batch Bioprocessing
SUPERVISOR: Nicole BORTH
PROJECT ASSIGNED TO: Larissa HOFER
The advancement of therapeutic protein and antibody biomanufacturing relies heavily on effective Chinese hamster ovary (CHO) cell bioprocessing as these cells are considered to be the staple mini-biofactories for recombinant protein production (Cordova et al., 2023). The structure of the target product strongly depends on cell culture conditions, that may be described by means of so-called critical quality attributes (CQAs), e.g., antibody glycosylation (Zhang et al., 2016). Consequently, it is imperative to implement rigorous control measures in the process, utilising appropriate critical process parameters (CPPs).(Brunner et al., 2016).
The first phase of the project focuses on a novel industrially relevant CHO cell line called “NIST-CHO”, which is publicly available to serve in future as a reference cell line to enable valid comparison of different optimisation and engineering strategies. This cell line will be fully characterised, employing advanced -omics techniques. This will allow the implementation of a fed-batch CHO cell bioprocesses under well-defined conditions. A Design of Experiments (DoE) approach will be adopted to systematically investigate various CPPs, e.g., pH, temperature, medium composition, or feeding strategies (Brunner et al., 2016). These parameters will be manipulated, monitored, and/or permuted to gain insights into their impact on CQAs. Through simultaneous monitoring of CQAs and CPPs, the project plans to implement hybrid models of the respective bioprocesses. Deploying these models, bioprocess parameters may be immediately adapted in the future in order to obtain the desired CQAs immediately in the course of the bioprocess.
In essence, the DigiTherapeutX initiative embodies a collaborative and multidisciplinary endeavor aimed at advancing the comprehension and utilisation of industrially significant CHO cell lines in bioprocessing, subsequently impacting the therapeutic protein production platform.
This project is funded by the FWF from the Austrian Science Fund (FG 1201-N). Collaboration partners of this project are the Paris Lodron University of Salzburg (research group Huber) and Technical University Vienna (research group Birner-Grünberger).

Figure 1 | Schematic of the implementation of multi-omics techniques into bioprocesses (Farrell et al., 2014).
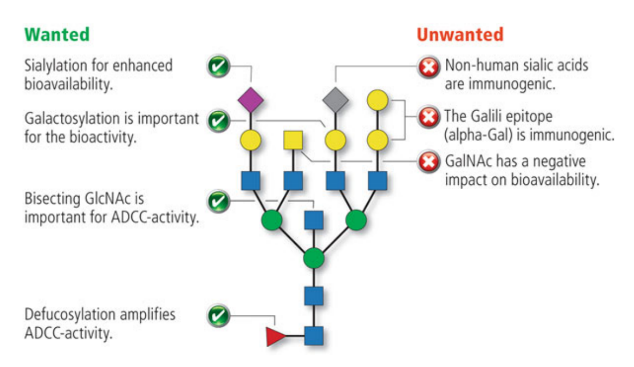
Figure 2 | Influence of the glycosylation pattern on the properties of monoclonal antibodies (Delobel, 2021).
References
Brunner, M. et al. (2016) ‘Investigation of the interactions of critical scale-up parameters (pH, pO2 and pCO2) on CHO batch performance and critical quality attributes’, Bioprocess and Biosystems Engineering, 40, pp. 251–263. Available at: doi.org/10.1007/s00449-016-1693-7.
Cordova, L.T. et al. (2023) ‘Generation of reference cell lines, media, and a process platform for CHO cell biomanufacturing’, Biotechnology and Bioengineering, 120(3), pp. 715–725. Available at: doi.org/10.1002/bit.28290.
Farrell, A. et al. (2014) ‘Application of Multi-Omics Techniques for Bioprocess Design and Optimization in Chinese Hamster Ovary Cells’, Journal of Proteome Research, 13(7), pp. 3144–3159. Available at: doi.org/10.1021/pr500219b.
Zhang, P. et al. (2016) ‘Challenges of glycosylation analysis and control: an integrated approach to producing optimal and consistent therapeutic drugs’, Drug Discovery Today, 21(5), pp. 740–765. Available at: https://doi.org/10.1016/j.drudis.2016.01.006.
